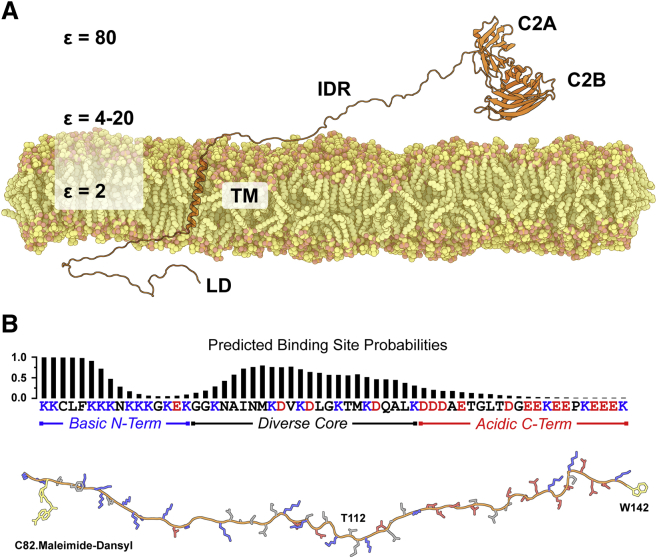
Figure 1.
Model of Syt 1 and amino acid sequence of its IDR. (A) Syt 1 consists of a short lumenal domain (LD), a single transmembrane helix (TM), a ∼60-residue IDR, and two calcium ion- and phospholipid-binding C2 domains in tandem, C2A and C2B. Approximate dielectric constants for the bilayer core (ε = 2), interfacial region (ε = 4–20), and bulk solution (ε = 80) are shown to indicate environments the polyampholytic IDR may experience. (B) The IDR sequence (residues 80–141) is shown with the basic residues in blue and the acidic residues in red. The distribution of charged residues partially delineates the sequence into three segments as indicated. Above the sequence is the ANCHOR-predicted probability for each residue potentially contributing to a binding site as described in (13, 14). The modeled peptide below the sequence shows the location for covalent attachment of acceptor dyes as well as location of the added tryptophan (yellow) used in FRET experiments. Thr112, the phosphorylation site examined in this study, is also indicated. To see this figure in color, go online.