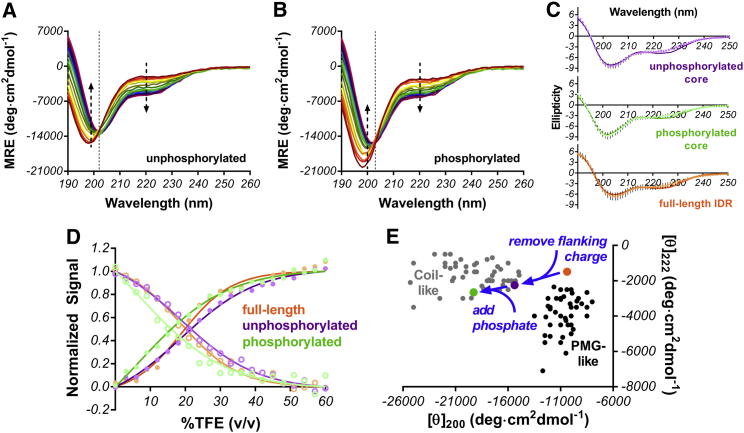
Figure 4.
Impact of Thr112 phosphorylation on Syt 1 IDR core region (GKNAINMKDVKDLGKTMKDQALKDDDAETGLTDG). (A) MRE of unphosphorylated Syt 1 IDR core and (B) MRE of phosphorylated Syt 1 IDR core is shown as a function of increasing TFE (0–60% v/v going from red to violet) in 10 mM sodium phosphate at a pH of 7.4. (C) Fitting of absorption profiles to linear combinations of α-helix, β-sheet, and random coil in each IDR construct is shown at a maximal TFE concentration of 60% v/v. (D) Simultaneous fitting is shown of the 198-nm coil minimum (solid circles, dark line) and 222-nm (open circles, lighter line) signals during folding transition for unphosphorylated (purple), phosphorylated (green), and full-length (orange) peptides. (E) Comparison of 222:200-nm ratio from full-length (orange), unphosphorylated core (purple), and phosphorylated core (green) is shown. Removal of flanking charge shifts IDR from its near premolten globule-like state to a more coil-like state and phosphorylation accentuates this effect. This plot was created using data reported in Ref. (34). To see this figure in color, go online.