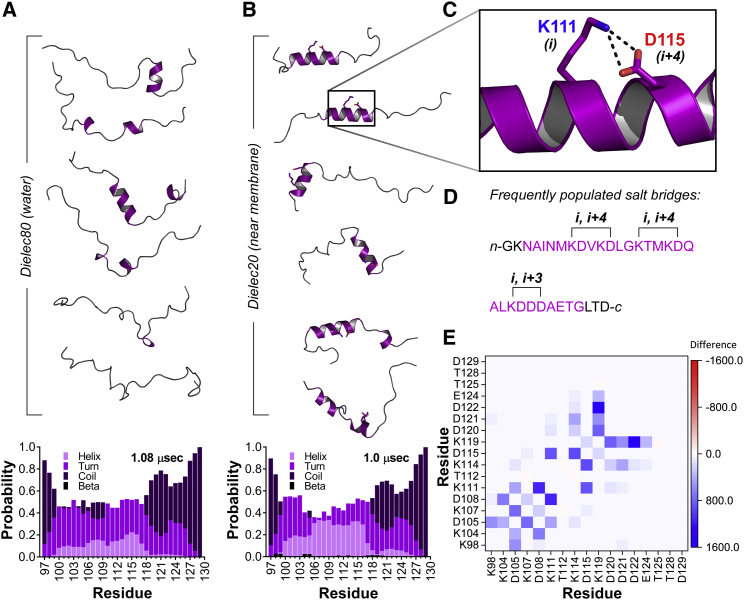
Figure 5.
Simulated structural ensemble of unphosphorylated Syt 1 IDR core region. (A) Representative conformers of the peptide at a dielectric constant of 80 with secondary structure probability per residue shown below (histogram). (B) Representative conformers of the peptide at a dielectric constant of 20 and secondary structure probability per residue shown below (histogram). Note increase in helical probability. (C) Closeup is given of example salt bridge that forms to stabilize helix in low dielectric environment. (D) IDR core region sequence shows sample salt-bridge interactions found in helices at a dielectric constant of ε = 20. Residues highlighted in purple occupy helical secondary structure. (E) Heat map shows change in all salt-bridge interactions in going from dielectric 80 to dielectric 20. An increased frequency of interaction between two charged residues is indicated by gradations of blue, whereas decreased interactions are indicated by gradations of red. Note increased frequency of i, i+3 and i, i+4 contacts. To see this figure in color, go online.