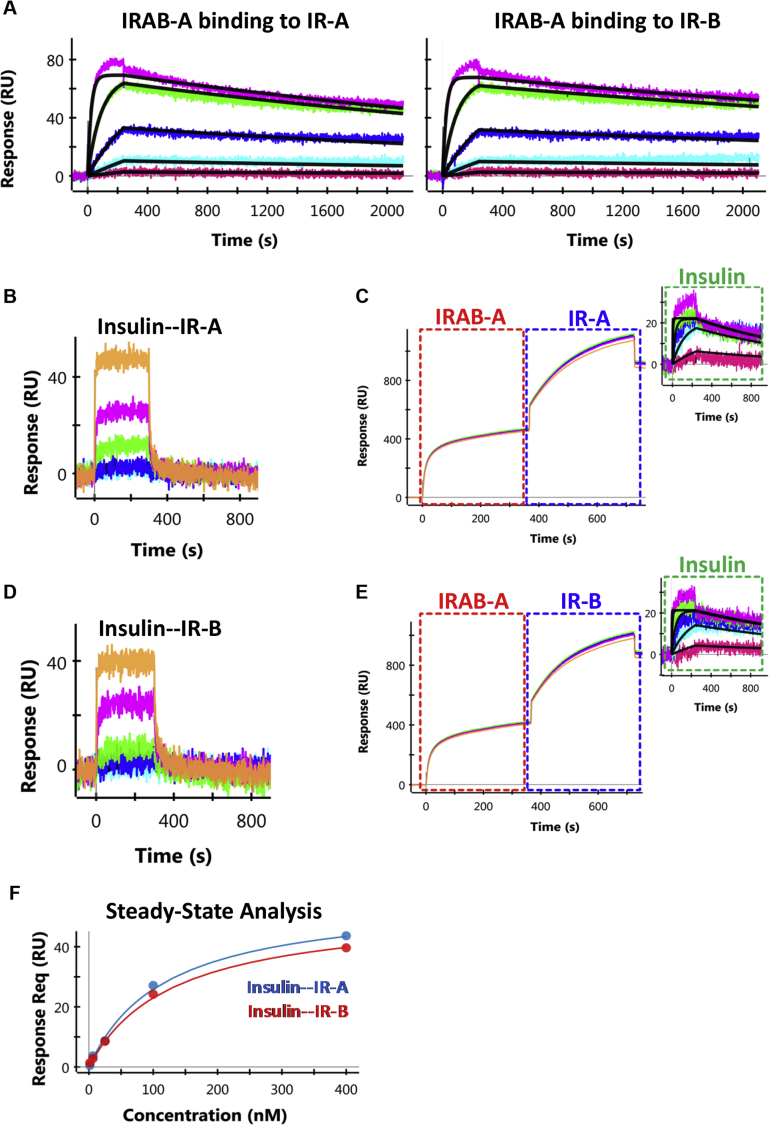
Figure 1.
SPR sensorgrams for IRAB-A or insulin binding to recombinant IR. (A) High affinity binding of IRAB-A to IR-A and IR-B. IRAB-A was captured using an anti-Fc chip, followed by the titration of IR (400 nM – 1.56 nM at 4-fold dilutions). A 1:1 Langmuir model (black lines) was used to fit the binding profiles. (B, D) Weak binding of insulin to IR-A and IR-B isoforms, respectively, observed in the absence of IRAB-A. The receptors were captured through their poly-histidine tag and was followed by the titration of insulin (400 nM – 1.56 nM at 4-fold dilutions). (C, E) Insulin binding to IR-A and IR-B isoforms, respectively, measured in the presence of IRAB-A. Capture of IRAB-A is indicated by the red boxes, followed by receptor capture (blue box), and finally, the titration of insulin (2000 nM – 3.2 nM at 5-fold dilutions, green box). A 1:1 Langmuir model (black lines) was used to fit the profiles of insulin binding to the IR/IRAB-A complex. (F) Binding analysis of insulin binding to IR-A and IR-B using steady-state equilibrium model.