Figure 2.
Impact of the Identified Variants on Protein Structure
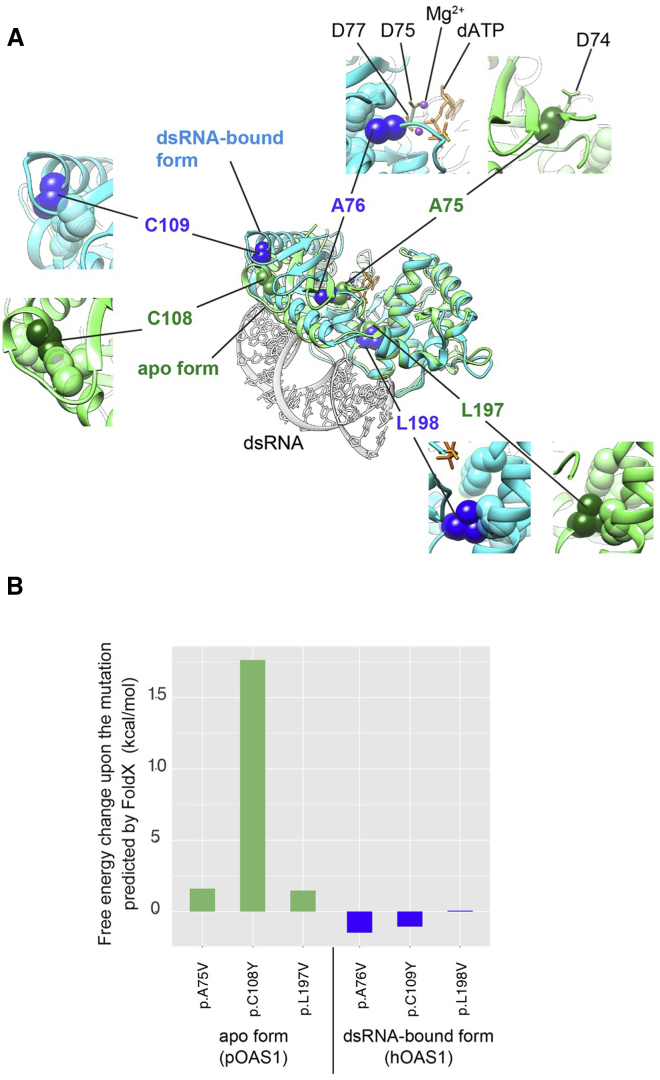
(A) Mapping of the mutations on the crystal structures of a double-stranded RNA (dsRNA)-bound form of human oligoadenylate synthetase 1 (hOAS1) and an apo form of porcine OAS1 (pOAS1) (PDB: 4ig8 and 1px5, respectively). The superimposed structures of the dsRNA-bound (cyan) and apo (green) forms of OAS1 are presented as ribbon diagrams. The stick model is shown in gray. The altered residues are shown as van der Waals spheres in blue and dark green in the dsRNA-bound and apo forms, respectively. The substrate analog, 2′-deoxy ATP (dATP), and magnesium ions are shown as orange sticks and purple balls, respectively. Close-up views around the mutation sites are shown separately for the dsRNA-bound and apo forms. In the close-up views, some side chains of the hydrophobic residues around the mutation sites are shown as translucent spheres.
(B) The free energy changes associated with each variant in a dsRNA-bound form of hOAS1 and apo form of pOAS1 by the FoldX software are shown.