Figure 2.
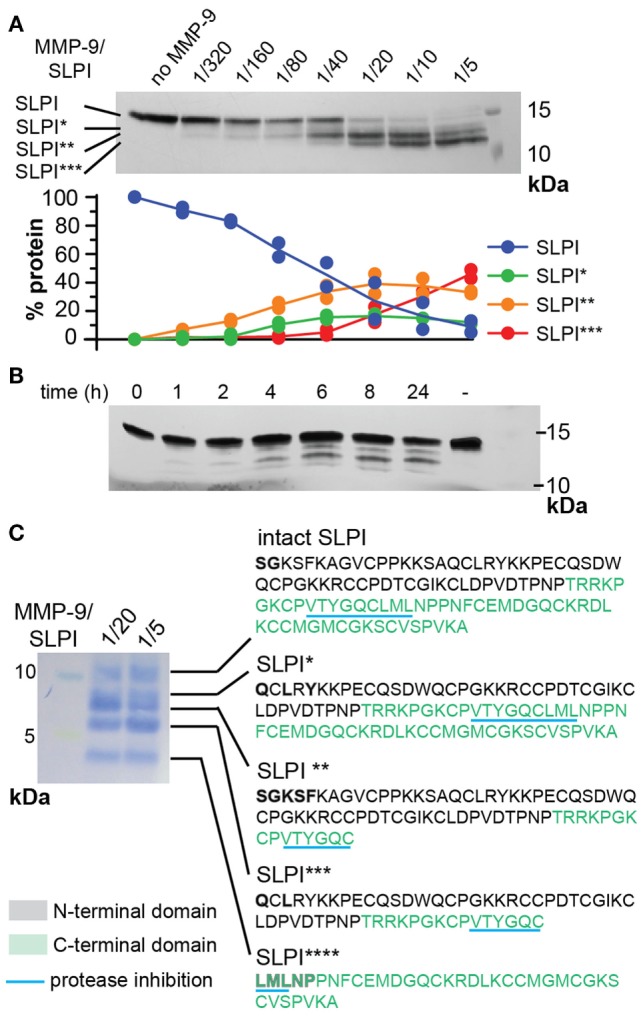
Cleavage of human secretory leukocyte peptidase inhibitor (SLPI) by matrix metalloproteases (MMP-9). (A); Incubation of SLPI with increasing quantities of active MMP-9 for 24 h at 37°C. Aside intact SLPI, three SLPI fragments were visualized; SLPI*, SLPI**, and SLPI*** (top). Densitometry analysis (bottom) of two independent experiments illustrates the appearance of fragments in the following order: SLPI**, SLPI*, and SLPI***. Data expressed as percentage of total SLPI protein. (B) Time-dependent incubation of SLPI with active MMP-9 (molar ratio 1/20). (C) Protein staining after blot transfer to a solid support and N-terminal sequencing of SLPI fragments. After blotting of larger quantities of SLPI, four cleavage fragments (SLPI*–SLPI****) were visible. N-terminal sequencing analysis revealed the presence of two MMP-9 digestion sites, also indicated in Figure 1. Amino acids retrieved by N-terminal sequencing are marked in bold. Amino acid color codes correspond with the N-terminal and C-terminal domains, as shown in Figure 1.
