Figure 6.
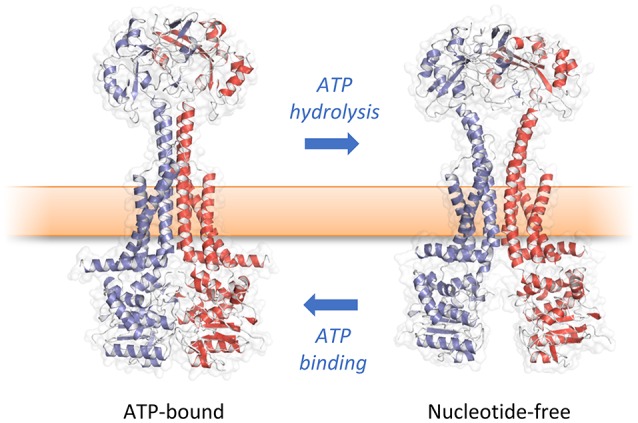
Mechanotransmission mechanism of MacB. ATP binding and hydrolysis cause large, transmembrane conformational changes in MacB structure. Rather than transporting substrates across the inner membrane, MacB-like proteins coordinate reversible dimerization of their NBDs with periplasmic conformational changes. TEP-forming MacB homologs use periplasmic conformational change to drive substrates across the bacterial outer membrane via TolC-like exit ducts. MacB homologs that do not form TEPs are proposed to use similar motions during lipoprotein trafficking and transmembrane signaling. Adapted from Crow et al. (2017).
