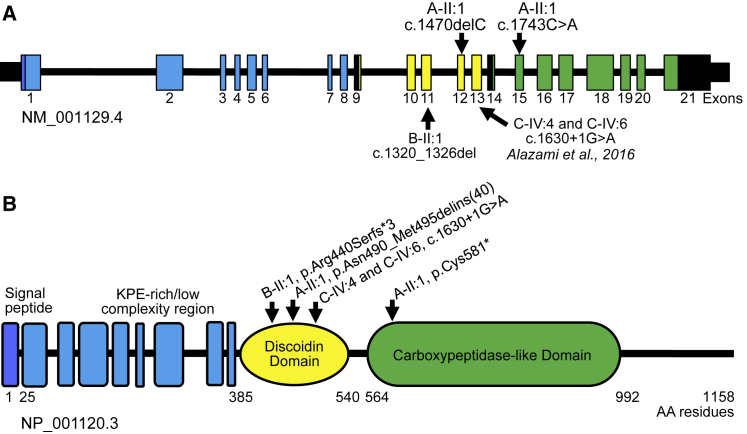
Figure 2.
Schematic Representation of AEBP1, ACLP Protein Structure, and Summary of Known Mutations
(A) Exome sequencing revealed compound heterozygous (A-II:1) and homozygous (B-II:1) nonsense and small deletion frameshift variants in AEBP1. The homozygous splice site variant described in two siblings (C-IV:4 and C-IV:6) with similar clinical features by Alazami et al.9 is also shown. AEBP1 encodes the collagen binding protein.
(B) Aortic carboxypeptidase-like protein (ACLP) has several distinct domains that are thought to mediate different protein-protein interactions including a central discoidin domain that helps mediate binding and specificity for fibrillar collagens. ACLP also contains a catalytically inactive metallocarboxypeptidase-like domain. Mutations identified fell within the discoidin domain or immediately downstream and included nonsense, frameshift, and canonical splice site variants that were predicted to result in loss of function.