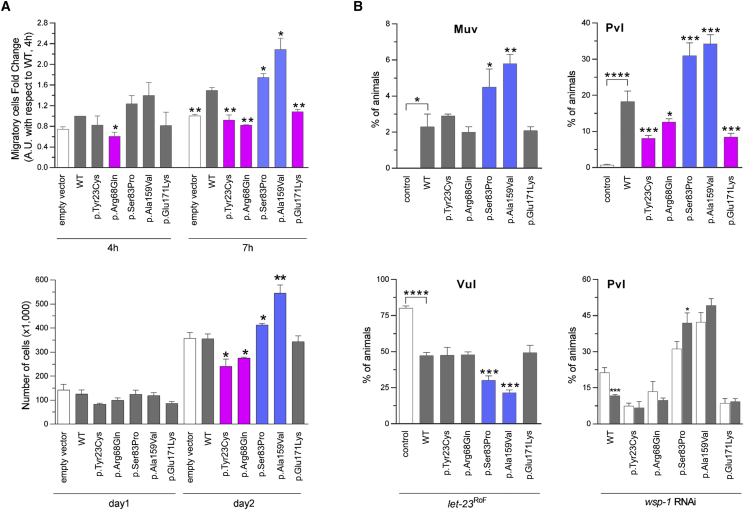
Figure 3.
In Vitro and In Vivo Functional Characterization of CDC42 Mutations
(A) CDC42 mutations differentially impact polarized migration and cell proliferation. Wound-healing assays (above) and proliferation assays (below) were performed using NIH 3T3 cells transiently transfected to express wild-type CDC42 or each of the indicated mutants. Mean ± SD densitometry values of three independent experiments are shown. The wound was generated 24 hr after transfection, and migration in the wounded area was evaluated after 4 and 7 hr. Cells expressing exogenous wild-type CDC42 migrate more rapidly into the scratched area than cells transfected with the empty vector (EV). Mutants differentially perturb polarized migration, with CDC42Ser83Pro and CDC42Ala159Val overexpression variably enhancing the wound closure ability of transfected cells compared to the wild-type protein, whereas CDC42Tyr23Cys, CDC42Arg68Gln, and CDC42Glu171Lys fail to do that, supporting a gain-of-function and a loss-of-function effect of these mutants, respectively. Cell proliferation was evaluated in transfected cells at the indicated time points and quantified by manual counting using a Neubauer hemocytometer. The trypan blue dye exclusion test was used to consider viable cells only. While the CDC42Ala159Val and CDC42Ser83Pro mutants variably enhance proliferation compared to cells expressing wild-type CDC42, no effect on proliferation (CDC42Glu171Lys) and reduced proliferation (CDC42Tyr23Cys and CDC42Arg68Gln) is documented for the other mutants, indicating a loss-of-function and a dominant-negative effect, respectively. Asterisks indicate significant differences compared with wild-type CDC42 (∗p < 0.05; ∗∗p < 0.01; Student’s t test).
(B) Consequences of CDC-42 expression on vulval development in C. elegans. Ectopic expression of wild-type CDC-42 at the L2/L3 stage elicits a multivulva (Muv) phenotype (left, upper panel), and CDC-42 overexpression in a LET-23/EGFR hypomorphic background reduces the penetrance of the vulvaless (Vul) phenotype (left, lower panel). Compared to animals expressing wild-type CDC-42, those expressing CDC-42Ser83Pro and CDC-42Ala159Val show higher prevalence of the Muv phenotype and lower prevalence of the Vul phenotype, indicating a gain-of-function role on LET-60/RAS signaling. Animals expressing the other tested CDC-42 mutants do not significantly differ from those expressing wild-type CDC-42. Ectopic expression of wild-type CDC-42 at the early L3 stage elicits a protruding vulva (Pvl) phenotype (right, upper panel). Animals expressing CDC-42Ser83Pro and CDC-42Ala159Val show a higher prevalence of the phenotype compared to worms expressing wild-type CDC-42, while a less penetrant phenotype was scored for animals expressing CDC-42Tyr23Cys, CDC-42Arg68Gln, or CDC-42Glu171Lys mutants. RNA interference (RNAi) experiments show that the Pvl phenotype associated with overexpression of wild-type CDC-42 is mediated, in part, by WSP-1/WASP (right, lower panel). White and gray bars indicate the penetrance of Pvl in non-interfered and interfered animals, respectively. Error bars indicate SEM of four independent experiments, and asterisks specify significance differences between animals expressing CDC-42 mutants and those expressing wild-type CDC-42 or between interfered and non-interfered nematodes (∗p < 0.05; ∗∗p < 0.001; ∗∗∗p < 0.0001; ∗∗∗∗p < 0.00005; two-tailed Fisher’s exact test). Comparisons between worms expressing wild-type CDC-42 and control animals are also shown. RNAi was performed by feeding using HT115 E. coli bacteria expressing double stranded wsp-1 RNA (Ahringer’s C. elegans RNAi feeding library) and optimized to overcome lethality. As a control of the efficiency of the modified RNAi protocol, let-60 RNAi experiments were performed on animals carrying the let-60 gain-of-function allele n1046 (p.Gly13Glu), and the prevalence of the Muv phenotype was scored at a dissecting microscope (Table S4).