FIGURE 2.
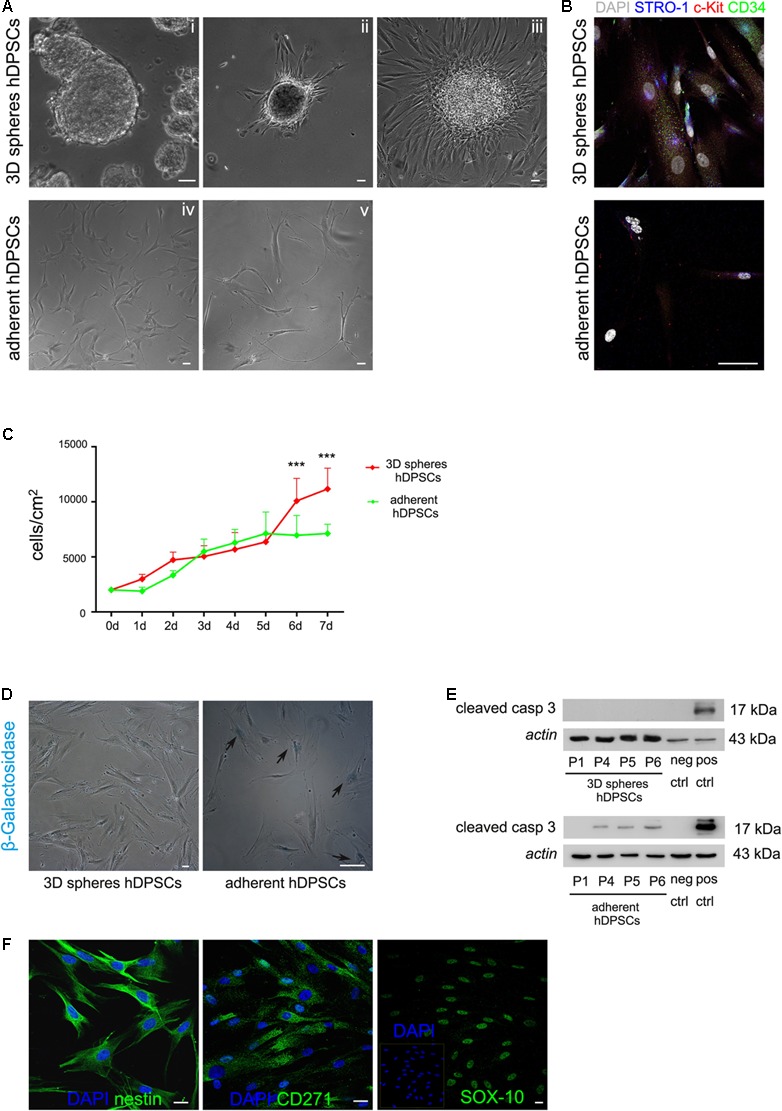
(A) Phase contrast images showing that hDPSCs, being cultured for repeated passages as floating three-dimensional spheres (i), attached to glass coverslips formerly treated with 0.005% poly-L-lysine (ii) and started to spread out of the spheres (iii), also keeping their fibroblast-like morphology. Human DPSCs cultured under adherent conditions showed a shift in cell morphology from the initial seeding (iv) up to passage 8 (v). (B) Immunofluorescence analysis showed the expression of the stemness markers c-Kit, STRO-1, and CD34 in hDPSCs derived from 3D sphere culture and in hDPSCs cultured in adherent conditions, following prolonged culture times. Bar: 50 μm. (C) Evaluation of proliferation ability in STRO-1+/c-Kit+/CD34+ hDPSCs cultured for 1 week, after 3D sphere culture and adherent culture conditions, respectively. Values were expressed as mean ± SD (∗∗∗P < 0.001 3D spheres hDPSCs vs. adherent hDPSCs). (D) Cell senescence was evaluated through the assay of β-galactosidase activity in 3D sphere-derived STRO-1+/c-Kit+/CD34+ hDPSCs and STRO-1+/c-Kit+/CD34+ hDPSCs cultured in adherent conditions, respectively, for 6 passages. Blue staining indicates cells positive for β-galactosidase activity (black arrows). Bar: 50 μm. (E) Western blot analysis of cleaved caspase 3 expression by STRO-1+/c-Kit+/CD34+ hDPSCs at different passages, following culture as 3D spheres and adherent culture conditions, respectively. Negative and positive controls of cleaved caspase 3 (Cell Signaling) were loaded on the right. Actin bands were presented as control of protein loading. (F) Immunofluorescence analysis was performed on STRO-1+/c-Kit+/CD34+ hDPSCs derived from 3D sphere culture, at late passages, to assess the expression of neural crest markers nestin, CD271 and SOX-10. Nuclei were counterstained with DAPI. Bar: 10 μm.
