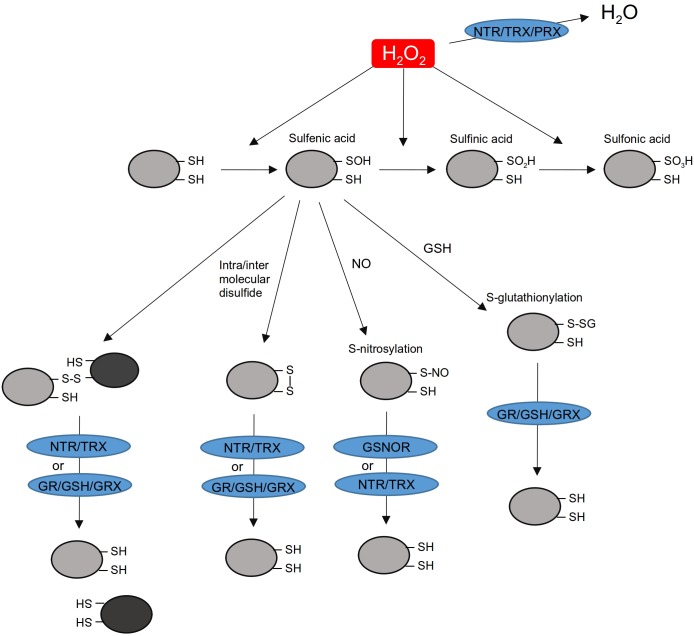
FIGURE 1.
H2O2-induced thiol modifications and scavenging activities in the nucleus. H2O2 accumulating in the nucleus can be detoxified by a NTR/TRX/PRX system. While many other H2O2 detoxification enzymes are active in plants (e.g., catalases and ascorbate peroxidases), their presence in the nucleus is not demonstrated yet. They are not represented here. H2O2 can oxidize thiol residues in protein. Sulfenic acid reacts with GSH, NO or with adjacent thiol residues. Putative nuclear proteins prone to S-glutathionylated, S-nitrosylated, or disulfide bonds formation have been identified by proteomic and biochemical approaches (see Supplementary Table 1; Delorme-Hinoux et al., 2016; Pérez-Pérez et al., 2017). S-glutathionylated, S-nitrosylated, or intra/intermolecular disulfide bonds can be reduced by NTR/TRX, GR/GSH/GRX, or GSNOR. NTR, NADPH-dependent thioredoxin reductase; TRX, thioredoxin; GR, glutathione reductase; GRX, glutaredoxin; PRX, peroxiredoxin; GSNOR, S-nitrosoglutathione reductase; GSH, glutathione; NO, nitric oxide; H2O2, hydrogen peroxide.