Figure 5.
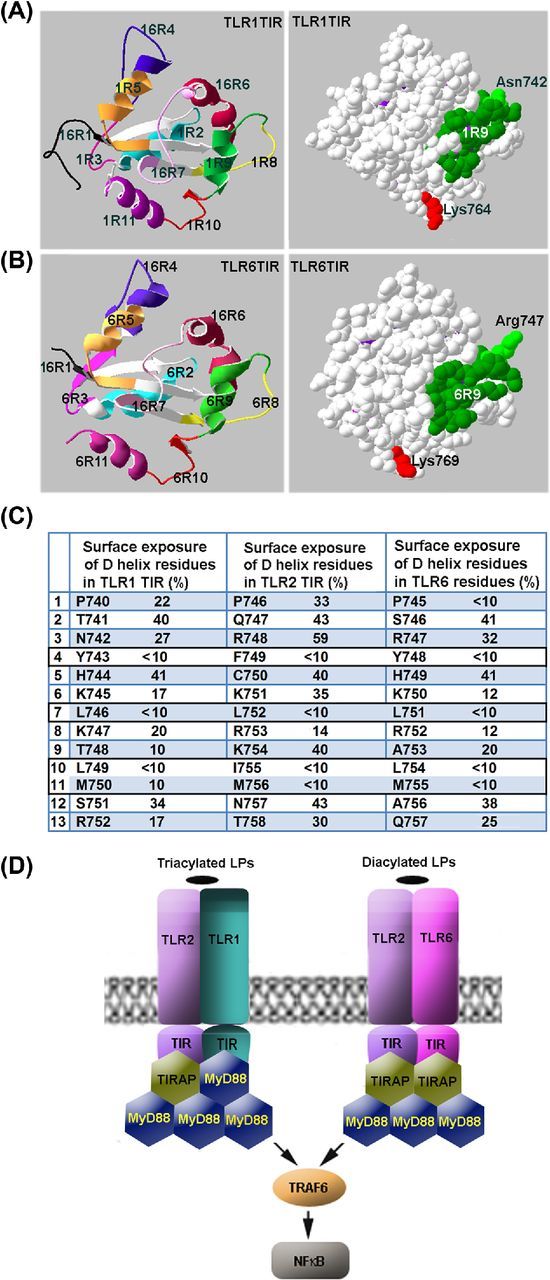
Mechanisms of adapter recruitment in TLR2 signaling. (A, B) TLR1 and TLR6 TIR domain regions that correspond to decoy peptides. (C) Surface exposure of region 9 residues in TIR domains of TLR1, TLR2 and TLR6. Surface exposure of the residues was calculated based on the available crystal structures of TIR domains of human TLRs. It should be noted that the D helix residues that have surface exposure <10% are 100% conserved not only in human TLR1 and TLR6, but also in mouse homologs of the proteins. (D) Schematic models of adapter recruitment to TLR2/1 and TLR2/6 heterodimers. The TLR2/1 heterodimer directly binds TIRAP and MyD88. The TLR2/6 heterodimers bind two TIRAP TIR domains through direct interactions mediated by D helices of TIR domains of both receptors. Additional MyD88 molecules are recruited to the primary complex through TIRAP-MyD88 and MyD88-MyD88 interactions. Disruption of any TIR–TIR interaction involved in the assembly of TLR signaling complexes abolishes signaling. Differences in composition of TLR2/1 and TLR2/6 complexes may account for kinetical differences in activation of NFκB and MAPKs by TLR2/1 and TLR2/6 agonists.
