Figure 3.
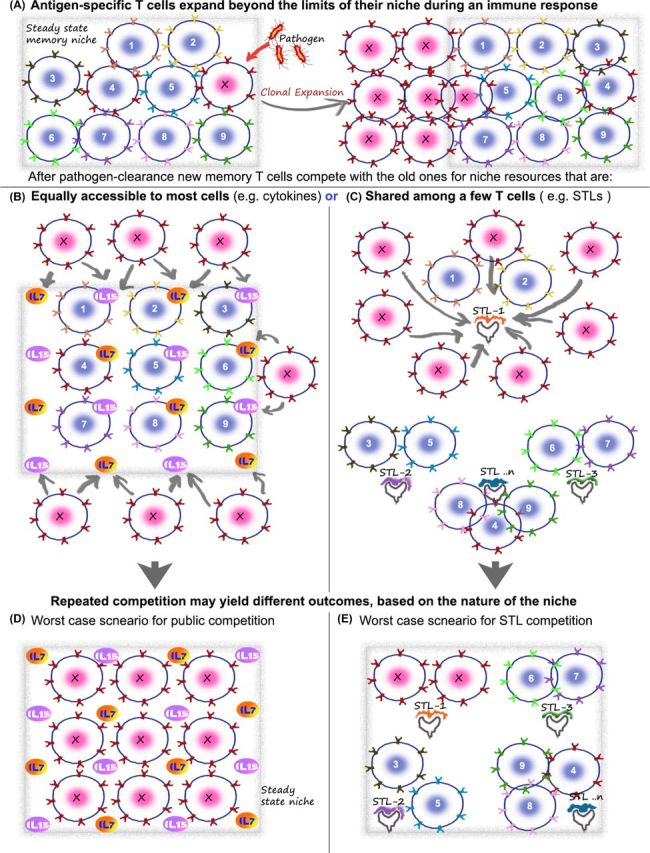
Potential consequences of public versus private competition on the diversity of memory T cells. The basic concept of memory maintenance on the basis of cytokines and STLs is cartooned here, using a hypothetical ‘worst-case’ scenario to illustrate my point that rampant competition between T cells without a restriction of niches can potentially have deleterious consequences on the repertoire. (A) Consider a memory repertoire with a diverse population of memory T cells that have accumulated over time in the individual (cartoon shows T cells 1–9 and X). The frequency of each is relatively low and this allows the total population to exist stably in the niches available in the immune system (niche boundaries marked by gray box). Upon pathogen entry, the appropriate T cell (here marked by X) expands to large numbers, which can even numerically be equal 10%–50% of the total repertoire. The key issue is what happens next. After the pathogen is cleared, this clone of T cells—expanded to large numbers—can go back to competition for resources in the immune niche. The potential drawback of using only public factors for this process is illustrated in (B) and (D), while the solution potentially offered by weak self-ligands (i.e. subthreshold ligands or STLs) is shown in (C) and (E). (B and D) Since X can stochastically compete with all other T cells (provided they have the receptors for the cytokine), it is possible that it can replace some of the rare memory T cells who are at a numerical disadvantage to the larger numbers of X. This can be costly, since the rare memory T cell that was lost in the process could represent a vital protective agency against a future infection by a less frequently appearing but debilitating pathogen. One way the number of new memory T cells that can compete for the cytokine niches (i.e. X competing for IL-7 and IL-15 here) is reduced is by regulating the expression of cytokine receptors on X. But even so, the large numbers of receptor positive cells that are available and the threat of repeated infections do offer a significant threat to the repertoire. Regardless of whether the total memory T-cell number at this point (after multiple infections) stays the same or slowly increases with time, it is important to note that only a small fraction of the effector cells are retained. So, a large number of the new ‘memory recruits’ can potentially compete with pre-existing memory T cells for their survival niches. As a result of this (shown in D), T-cell clones remaining after the recent infection can occupy every niche—theoretically capable of displacing in a stochastic fashion most of the other pre-existing memory T cells in the process. If the pool size can grow a little, this is unlikely to be a serious concern. However, over time the repertoire will be disproportionally biased toward clones remaining from the most repetitive infections—e.g. seasonal flu as opposed to rarer (and potentially more deadly) infections. (C) In the case of competitive niches defined by STLs (in addition to or instead of cytokines), the new recruits (X) only compete with a specific subset of T cells that engage that particular STL. If the diversity of STLs is large enough, each niche is likely to be quite small. Unlike the fixed scope for sources of IL-7/15, etc. in the body, the niche of STLs can be expanded—by using new sources of self-ligands or commensal antigens as the individual's repertoire grows. In this case (cartooned in E), even after repeated responses by a clone, in the ‘worst-case’ scenario, replacement of all the other receptors in a particular niche is unlikely to make a serious dent in the complexity of the total repertoire. As discussed in the text, since multiple TCRs specific for the same antigen can segregate randomly across multiple STLs, the repertoire is likely to retain a broad suite of T cells representative of the individual's pathogen exposures, with this mechanism.
