Fig. 1.
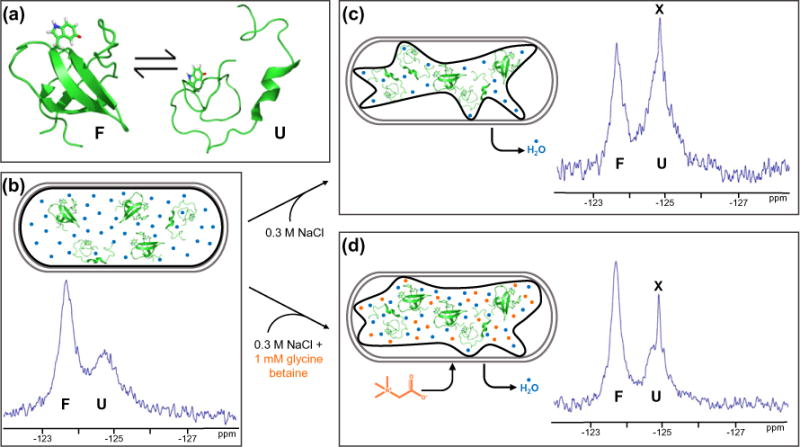
Glycine betaine reverses the destabilizing effect of hyperosmotic shock. (a) SH3 exists in an equilibrium between its folded state (PDB ID: 2A36) and an unfolded ensemble with a free energy of unfolding near zero under non-denaturing conditions. Tryptophan 36 with fluorine at position 5 is highlighted in red. Protein stability was measured in live E. coli cells under three conditions at 298 K. (b) Both the unfolded and folded forms are populated in cells under normal osmotic conditions. Gray outlines represent the cell wall. Black outlines represent the cytoplasmic membrane. Blue circles represent H2O. (c) Hyperosmotic shock caused by adding 0.3 M NaCl to the media destabilizes SH3. (d) Adding 1 mM glycine betaine to the 0.3 M NaCl causes the uptake of glycine betaine, returning SH3 to the stability observed without osmotic shock. Orange circles represent glycine betaine. Leakage of fluorine-containing metabolites (X) occurs upon hyperosmotic shock (Fig. S1).
