Table 1.
Optimization of Cr-catalyzed cyclopropanation.
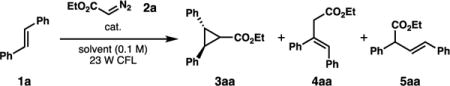
| |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| entry | cat. (mol%) | solvent | 2a equiv | time (h) | NMR yield (%)a | ||
| 3aa | 4aa | 5aa | |||||
| 1b | Cr1 (1) | CH3NO2 | 5 | 24 | 60 | 8 | 7 |
| 2 | Cr1 (1) | CH3NO2 | 5 | 24 | 61 | 10 | 8 |
| 3 | Cr1 (1) | CH3NO2 | 1.1 | 40 | 60 | 8 | 11 |
| 4 | Cr2 (1) | CH3NO2 | 1.1 | 40 | 5 | 1 | 1 |
| 5 | Cr3 (1) | CH3NO2 | 1.1 | 40 | 40 | 8 | 7 |
| 6 | Cr4 (1) | CH3NO2 | 1.1 | 40 | 50 | 9 | 8 |
| 7 | Ru1 (1) | CH3NO2 | 1.1 | 24 | 3 | 0 | 0 |
| 8 | Ru1 (1) | CH3NO2 | 5 | 14 | 9 | 4 | 5 |
| 9 | Ru2 (5) + MV (15)c | CH3NO2 | 5 | 21 | 12 | 2 | 2 |
| 10 | Ru2 (5) | CH3NO2 | 5 | 21 | 0 | 0 | 0 |
| 11 | Cr1 (1) | CHCl3 | 1.1 | 24 | 18 | 1 | 2 |
| 12 | Cr1 (1) | acetone | 1.1 | 24 | 57 | 5 | 8 |
| 13 | Cr1 (1) | CH2Cl2 | 1.1 | 24 | 73 | 2 | 7 |
| 14 | Cr1 (1) | CH3CN | 1.1 | 24 | 67 | 8 | 10 |
| 15 | Cr1 (1) | DCE | 1.1 | 24 | 73 | 0 | 5 |
| 16 | none | DCE | 1.1 | 14 | 0 | 0 | 0 |
| 17 | CrCl3 (10) | DCE | 1.1 | 49 | 0 | 0 | 0 |
| 18d | Cr1 (1) | DCE | 1.1 | 49 | 0 | 0 | 0 |
Determined using veratraldehyde as a standard.
Near UV light (bulbs at 300, 350, and 419 nm) used instead of 23 W CFL.
MV: methyl viologen2+·(PF6)2.
Reaction performed in dark (foil wrapped).
