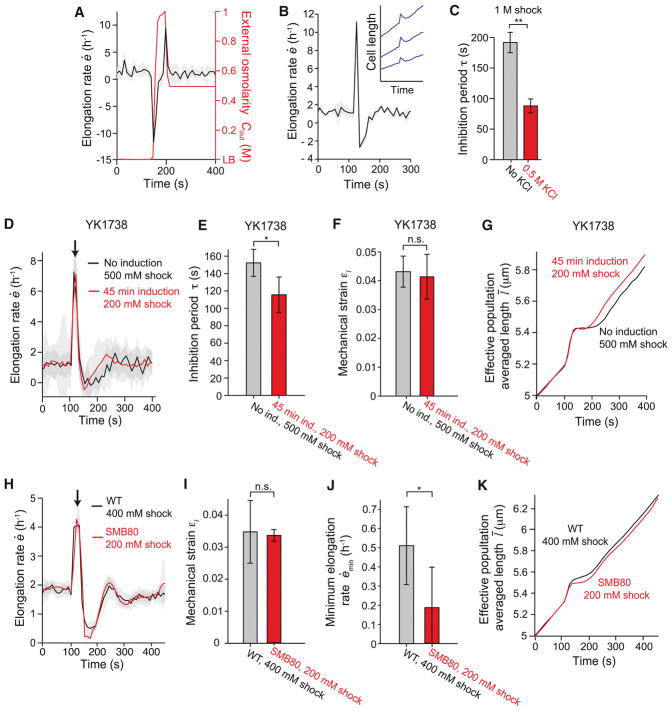
Figure 5. Cells producing membrane at an accelerated rate display attenuated growth inhibition.
(A) Population-averaged elongation rate of WT cells in response to a 1-M hyperosmotic shock followed by a 500-mM hypoosmotic shock does not display growth inhibition (n = 7). (B) In the presence of 500 mM KCl, population-averaged elongation rate in response to a 1-M hypoosmotic shock becomes negative, indicating cell shrinkage (n = 3). Inset: length of three cell chains during the same shock. (C) Inhibition period in response to a 1-M hypoosmotic shock decreases in the presence of 500 mM KCl (n = 21 and n = 3 for 0 and 0.5 M KCl, respectively). **: Student’s t-test, p < 10−5. (D) Population-averaged elongation rate of B. subtilis YK1738 with wild-type levels of AccDA (black line) and induction of AccDA overexpression (red line) during hypoosmotic shock (n = 21 and n = 164 cell chains for uninduced and induced, respectively). Shock magnitudes (500 mM and 200 mM, respectively, from LB + 1 M sorbitol) were chosen to yield approximately equal mechanical strains. Shading indicates ±1 s.d. (E) The population-averaged mechanical strain of B. subtilis YK1738 was similar between populations with wild-type levels of AccDA and with induction (“ind.”) of AccDA overexpression during the hypoosmotic shocks in (D). *: Student’s t-test, p < 0.005. (F) The population-averaged ihibition of B. subtilis YK1738 was similar between populations with wild-type levels of AccDA and with induction (“ind.”) of AccDA overexpression during the hypoosmotic shocks in (D). n.s.: Student’s t-test, not significant. (G) Effective population-averaged length of B. subtilis YK1738 with induction of AccDA overexpression (red line) illustrates the shorter inhibition period relative to wild-type levels of AccDA during hypoosmotic shock. (H) Population-averaged elongation rate of wild-type B. subtilis JH642 (black line) and B. subtilis SMB80 lacking stretch-activated ion channels (red line) during hypoosmotic shock from LB + 500 mM sorbitol (n = 19 and n = 20 chains for WT and SMB80, respectively). Shading indicates ±1 s.e.m. (I) The mechanical strain induced in wild-type cells by a 400-mM hypoosmotic shock was similar to that of SMB80 cells by a 200-mM shock. Error bars indicate ±1 s.d. n.s.: Student’s t-test, not significant. (J) The minimum elongation rate caused in wild-type cells by a 400-mM hypoosmotic shock was lower than that caused in SMB80 cells by a 200-mM shock. Error bars indicate ±1 s.d. *: Student’s t-test, p < 0.005. (K) Effective population-averaged length of WT and SMB80 illustrates the lower minimum elongation rate of SMB80 cells during hypoosmotic shock. See also Figure S3.