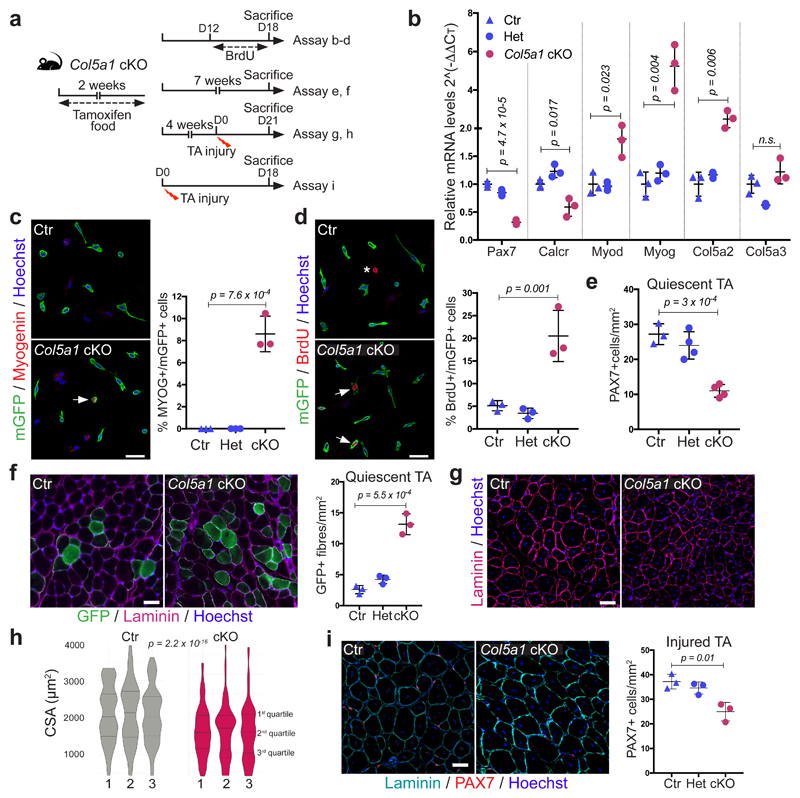
Figure 3. Satellite cell-produced COLV is required in vivo for self-renewal and maintenance of quiescence.
(a) Experimental schemes for Control (Ctr): Tg:Pax7-CT2;Col5a1+/+;R26mTmG, heterozygous (Het): Tg:Pax7-CT2;Col5a1flox/+;R26mTmG and conditional knock-out (cKO): Tg:Pax7-CT2;Col5a1flox/flox;R26mTmG mice.
(b) RT-qPCR of satellite cell (Pax7, Calcr) and differentiation (Myod, Myog) markers on Col5a1 mutant and control satellite cells isolated by FACS from resting muscle (n=3 mice/genotype).
(c) Representative images of membrane-GFP+ satellite cells from total muscle preparations from Control and Col5a1 null mice plated for 12h. Arrow, mGFP+/Myogenin+ cell (n=3 mice/genotype, ≥200 cells).
(d) GFP+ satellite cells from total muscle preparations plated for 12h. Asterisk, non-recombined BrdU+ cell; arrows, GFP+/BrdU+ cells (n=3 mice/genotype, ≥250 cells).
(e) Satellite cell quantification in Control, Het and Col5a1 cKO mice 7 weeks after tamoxifen treatment (n=3 (Ctr) and 4 (Het/cKO) TA muscle/genotype).
(f) Immunostaining of sections from Control and Col5a1 cKO TA muscles 7 weeks following tamoxifen treatment (n=3 mice/genotype).
(g) Immunostaining of sections from Control and Col5a1 cKO TA muscles 21d post-injury (n=3 mice/genotype).
(h) Muscle cross sectional area (CSA) distribution 21d post-injury showed as violin plots was significantly different in Control vs Col5a1 based on Kruskal-Wallis test (n=3 mice/genotype, 1000 fibres analysed/mouse).
(i) Immunostaining of sections 18 days post-cardiotoxin injury of Control and Col5a1 cKO TA muscles (n=3 mice/genotype).
Error bars, mean ± SD; two-sided unpaired t-test. Scale bar: 50μm, c and d; 100μm, f, g and i.