Abstract
Previous studies show Src family kinase (SFK) activation is involved in a response that stimulates Na,K-ATPase. Here, we tested whether SFK activation is involved in the Na,K-ATPase response to endothelin-1 (ET-1). Intact porcine lenses were exposed to 100 nM ET-1 for 5–30 min. Then, the epithelium was removed and used for Na,K-ATPase activity measurement and Western blot analysis of SFK activation. Na,K-ATPase activity was reduced by 30% in lenses exposed to ET-1 for 15 min. The response was abolished by the SFK inhibitor PP2 or the ET receptor antagonist, PD145065. Activation of a ~61 kDa SFK was evident from an increase in Y416 phosphorylation, which reached a maximum at 15 min ET-1 treatment, and a decrease in Y527 phosphorylation. PP2 prevented SFK activation. Since Fyn, Src, Hck, and Yes may contribute to the observed 61 kDa band, these SFKs were isolated by immunoprecipitation and analyzed. Based on Y416 phosphorylation, ET-1 appeared to activate Fyn, while Src and Hck were inhibited and Yes was unaltered. ET-1 requires SFK activation to cause Na,K-ATPase inhibition. ET-1 elicits a different pattern of SFK activation from that reported earlier for purinergic agonists that stimulate Na,K-ATPase activity and activate Src. In the ET-1 response Src is inhibited and Fyn is activated. The findings suggest SFK phosphorylation is involved in a regulatory mechanism for Na,K-ATPase. Knowing this may help us understand drug actions on Na,K-ATPase. Faulty regulation of Na,K-ATPase in the lens could contribute to cataract formation since an abnormal sodium content is associated with lens opacification.
Endothelin-1 (ET-1), best known as a vasoconstrictor, changes Na,K-ATPase (Na–K pump) activity in a number of tissues including renal proximal tubule, aorta, and capillary endothelium (Prasanna et al., 2001; Krishnamoorthy et al., 2003; Liu et al., 2009). In cardiac myocytes, ET-1 activates Src family tyrosine kinases (SFKs; Kovacic et al., 1998), a response thought to be a step in a hyprertrophic response. The ability of ET-1 to activate SFKs is interesting because in some tissues regulation of Na,K-ATPase activity occurs via tyrosine phosphorylation-dependent pathways (Sandiford et al., 2005; El-Beialy et al., 2010). Tyrosine phosphorylation and deposphorylation appear to be involved in the regulation of lens Na,K-ATPase in the eye (Bozulic et al., 2004). In the lens epithelium different SFKs were found to elicit different patterns of tyrosine phosphorylation and some SFKs caused a change in Na,K-ATPase activity (Bozulic et al., 2005). SFKs have been proposed as critical elements in certain G-protein-coupled receptor responses (Ma et al., 2000; Luttrell and Luttrell, 2004) and in rabbit lenses, SFK activation was found to be a required step in the chain of events responsible for a rapid increase of Na,K-ATPase activity in the epithelium that occurs following purinergic P2Y receptor activation (Tamiya et al., 2007). When the rabbit lens was exposed to ATP or UTP, Src was the main SFK activated.
The eye lens is a structure formed almost entirely from tightly packed elongated fiber cells and regulation of cytoplasmic sodium and potassium concentration is critical for transparency. Different regions of the lens display different Na,K-ATPase activity (Delamere and Dean, 1993). Fiber cells have low or negligible Na,K-ATPase activity. A monolayer of epithelium which covers the anterior surface maintains a high Na,K-ATPase activity and plays a key role in determining sodium and potassium concentrations in the entire lens (Delamere and Tamiya, 2009). The lens expresses several different G-protein-coupled receptors (Collison and Duncan, 2001; Lurtz and Louis, 2007). There is evidence for ET receptors along with purinergic receptors (Okafor et al., 2002). While purinergic agonists increase the rate of Na,K-AT Pase-mediated active 86Rb transport by the intact lens (Tamiya et al., 2007), the rate is reduced by ET-1 (Okafor and Delamere, 2001; Okafor et al., 2002). The two agonists elicit opposite effects on the rate of active Na–K transport. Interestingly, the effects of ET-1 on Na,K-ATPase-mediated active transport were abolished by the nonselective tyrosine kinase inhibitor genistein. The findings suggested ET-1 exposure inhibits Na, K-ATPase activity in lens epithelium by a tyrosine phosphorylation-dependent mechanism. Knowing SFK activation is involved in a response that stimulates Na,K-ATPase we were keen to learn whether SFK activation could be involved in a response that causes Na,K-ATPase inhibition. We hypothesized that ET-1 might trigger a different pattern of SFK activation from that caused by ATP and UTP.
Materials and Methods
Materials
Endothelin-1 was obtained from Phoenix Pharmaceuticals, Inc. (Burlingame, CA), and PP2 was obtained from EMD Chemicals, Inc. (Gibbstown, NJ). PD145065 was purchased from Enzo Life Sciences (Plymouth Meeting, PA). The following antibodies at the specified dilutions were used for Western blot analysis: mouse monoclonal anti Src (L4A1) antibody (Cell Signaling Technology, Danvers, MA) 1:1,000; rabbit polyclonal Phospho Src family (Tyr 416) antibody (Cell Signaling Technology) 1:1,000; rabbit polyclonal anti b-actin antibody (Cell Signaling Technology) 1:3,000; mouse monoclonal anti Hck unconjugated antibody (Abcam, Cambridge, MA) 1:1,000; mouse monoclonal anti-Yes unconjugated antibody (BD Bio Sciences, San Jose, CA) 1:2,000. The antibodies used for immunoprecipitation were: mouse monoclonal anti-Src (Clone GD11) agarose conjugate (Millipore, Billerica, MA; 8 μg/500 μg protein); mouse monoclonal anti-Fyn unconjugated antibody (Santacruz Biotechnology, Santacruz, CA; 1:20); mouse monoclonal anti Hck unconjugated antibody (Abcam; 1:20); mouse monoclonal anti-Yes unconjugated antibody (BD Bio Sciences; 1:20). Reagents for antibody conjugation in immunoprecipitation studies were: ethanolamine, triethanolamine, and dimethyl pimelimidate dihydrochloride (Sigma, St Louis, MO) and protein G agarose (Thermo Fisher Scientific, Rockford, IL). Protein G agarose beads for antibody conjugation were obtained from Thermo Fisher Scientific.
Lenses
Adult porcine eyes were obtained from Hatfield Quality Meats (Philadelphia, PA) and shipped overnight on ice. The use of porcine eye tissues was approved by the University of Arizona Institutional Animal Care and Use Committee and conformed to the ARVO Resolution for the Use of Animals in Ophthalmic and Vision Research. The posterior of the eye was dissected, and the zonules were cut. The lens were removed from the globe and placed in short-term organ culture (1 h) in Krebs’ solution at 37°C to equilibrate. The composition of the Krebs’ solution was (mM) 119 NaCl, 4.7 KCl, 1.2 KH2PO4, 25 NaHCO3, 2.5 CaCl2, 1 MgCl2, and 5.5 glucose at pH 7.4.
Measurement of ATPase activity
Intact porcine lenses were kept in organ culture and treated with ET-1 for 0, 5, 10, 15, 20, or 30 min. Control lenses (0 min) received only the vehicle. Lens epithelium, attached to the capsule (capsule–epithelium), was then isolated and immediately frozen in liquid nitrogen and stored at −80°C. The frozen epithelium samples were homogenized in an ice-cold 2× ATPase assay buffer comprising (mM): L-histidine, 80; NaCl 200; KCl, 10; MgCl2, 6.0; EGTA, 2.0 (pH 7.4) plus protease inhibitors; one complete mini protease inhibitor cocktail tablet for each 7 ml of ATPase buffer (Roche Applied Science, Mannheim, Germany). Entire capsule– epithelium from two lenses was homogenized in 400 μl of the 2× ATPase buffer. Homogenization was carried out for 1 min (4 strokes of 15 sec at 5 sec interval to avoid overheating the sample) using Misonix S3000 sonicator at 6W power setting (Misonix, New York, NY). The homogenate was centrifuged at 14,000g for 30 min at 4°C to remove nuclei and unbroken debris and the supernatant was used for measuring Na,K-ATPase activity. Protein in the homogenate was measured by the bicinchoninic acid (BCA) assay (Pierce Biotechnology, Rockford, IL; Smith et al., 1985), using bovine serum albumin as a standard.
Na,K-ATPase activity was measured using a modification of a published technique (Shahidullah and Delamere, 2006). Homogenate (150 μl) obtained from treated or control lens epithelium was added in duplicate tubes containing 50 μl of 2 ice-cold Na,K-ATPase buffer. To ensure access of ions and ATP to membrane vesicles, alamethicin (5 μl) was added to each tube to give a final approximate concentration of 0.1 mg of alamethicin per mg of protein (Xie et al., 1989). Ouabain, a specific inhibitor of Na,K-ATPase (Wallick and Schwartz, 1988), was added to half the tubes at a final concentration of 150 μM (5 μl), the remainder of the tubes received 5 μl of distilled water to equalize volume. Additional 150 μl distilled water was added to each tube so that the total volume of the assay mixture becomes 400 μl including the 40 μl ATP solution to be added to start the reaction. The tubes were preincubated at 37°C with gentle agitation for 5 min and then ATP (40 μl) was added to each tube to give a final concentration of 2.0 mM. The ATP hydrolysis reaction was allowed to proceed for 30 min at 37°C. The reaction was stopped by adding 150 μl of 20% ice-cold trichloroacetic acid (TCA) and placing the tubes on ice.
ATP hydrolysis was quantified by measuring the amount of inorganic phosphate released in each reaction tube. The tubes were centrifuged at 3,000 rpm for 15 min at 4°C then 400 μl of the supernatant was removed and added to 400 μl of 4.0% FeSO4 solution in ammonium molybdate (1.25 g of ammonium molybdate in 100 ml of 2.5 N sulphuric acid). NaH2PO4 standard solutions (equivalent to 0, 62.5, 125, 250, and 500 nmoles of PO4) were treated similarly. The mixture was allowed to sit for 10 min at room temperature then absorbance was measured at 750 nm using a plate reader (Victor V1420-040, Perkin Elmer, Branford, CT). Na,K-ATPase activity was calculated as the difference between ATP hydrolysis in the presence and in the absence of ouabain. ATPase activity was expressed as nmoles ATP hydrolyzed per milligram protein per 30 min.
Western blot analysis
Intact lenses were incubated in Krebs’ solution in the presence or absence of ET-1 (final concentration 100 nM) for a specified time (0, 5, 10, 15, 20, or 30 min). Then the capsule–epithelium was isolated from each lens. For Western blot analysis the capsule– epithelium was placed in ice-cold lysis buffer that contained (mM) 50 HEPES, 150 NaCl, 1 EDTA, 10 sodium fluoride, 10 sodium pyrophosphate, 2 sodium orthovanadate, 10% glycerol, 1% Triton X-100, 1% sodium deoxycholate, and protease inhibitors, one complete mini protease inhibitor cocktail tablet for each 7 ml (Roche Applied Science) and the pH adjusted to 7.5. Epithelium from two lenses was homogenized in 400 μl of the lysis buffer. Homogenization was carried out for 1 min (4 strokes of 15 sec at 5 sec interval) using Misonix S3000 sonicator at 6W power setting (Misonix). The homogenate was centrifuged at 14,000g for 30 min at 4°C to remove nuclei and unbroken debris and the supernatant was used for Western blot analysis. Sixty micrograms of protein was separated on a 7.5% SDS–PAGE and transferred to a nitrocellulose membrane. The membrane was treated with blocking buffer (LICOR, Lincoln, NE) for overnight at 4°C and incubated overnight at 4°C with one or more of the following: rabbit polyclonal anti-PY416 SFK antibody or rabbit polyclonal anti-PY527 Src, mouse monoclonal anti-Yes unconjugated antibody, mouse monoclonal anti Src (L4A1) antibody, mouse monoclonal anti Hck unconjugated antibody, mouse monoclonal anti-Fyn unconjugated antibody, and rabbit polyclonal anti-b-actin antibody. The anti-PY416 Src antibody cross-reacts with SFK members phosphorylated at the active loop tyrosine (PYA-SFK). The anti-PY527 Src antibody cross-reacts with SFK members phosphorylated at the COOH-terminal inhibitory tyrosine (PYT-SFK). After three 5-min washes with Tris-buffered saline + Tween 20, the nitrocellulose membrane was incubated for 60–90 min with anti-rabbit secondary antibody conjugated with IRDye 680 (LICOR) or anti-mouse secondary antibody conjugated with IR Dye 800 (LICOR). The membrane was washed three times with Tris-buffered saline + Tween 20 and three times with PBS. The bands were detected and quantified using an Odyssey infrared scanner (LICOR), capable of detecting two wavelengths simultaneously (680 and 800 nm).
Immunoprecipitation
The capsule–epithelium was placed in ice-cold RIPA (radioimmunoprecipitation assay) buffer that contained (mM) 50 HEPES, 150 NaCl, 1 EDTA, 10 sodium fluoride, 10 sodium pyrophosphate, 2 sodium orthovanadate, 10% glycerol, 0.5% Triton X-100, 0.5% Nonidet P-40, 0.5% SDS, phosphatase inhibitor cocktail 1 and 2 (1:100 each) (Sigma), and protease inhibitors; one complete mini protease inhibitor cocktail tablet for each 7 ml of ATPase buffer (Roche Applied Science) and the pH adjusted to 7.5. The capsule–epithelium was homogenized and centrifuged to remove debris as in the case of Western blot samples. In case of Src immunoprecipitation, 500 μg of total protein was incubated overnight at 4°C with the immunoprecipitation antibody: mouse monoclonal anti-Src antibody (clone GD11) conjugated to agarose beads (8 μg). For Yes, Hck, and Fyn immunoprecipitation, unconjugated antibodies were first cross-linked to immobilized Protein G agarose beads (1:20 v/v) following the manufacturer’s (Abcam) protocol. Five hundred micrograms of protein samples were then added to the conjugated antibody-agarose beads (50 μl) and incubated overnight at 4°C on an end-to-end hematology mixer at 12 rpm (Fisher Scientific, Pittsburgh, PA). The beads and immune complexes were collected by brief (1 min) centrifugation (5,000g) and washed three times with PBS. SDS sample buffer was added to the beads and incubated at 60°C for 20 min. Beads were pelleted by centrifugation (5,000g, 1 min) and the supernatant was used for Western blot analysis.
Statistical analysis of data
Results were expressed as the mean ± SEM of data from a specified number of independent experiments. Statistical comparisons were made by one-way analysis of variance followed by the Bonferroni post hoc multiple comparison test. A probability (P) value of <0.05 was considered significant.
Results
Effect on Na,K-ATPase activity
Intact organ-cultured porcine lenses were exposed to ET-1 (100 nM) for 5–30 min. Over 5 min Na,K-ATPase activity diminished in the epithelium removed from lenses exposed to ET-1. After 15 min Na,K-ATPase activity was approximately 30% less than the activity measured in the epithelium of control lenses that had been incubated in the absence of ET-1 (Fig. 1). To examine the role of SFKs in the Na,K-ATPase response, a different group of lenses was exposed to the specific SFK inhibitor PP2 (10 μM). In the presence of PP2, the Na,K-ATPase activity inhibition response to ET-1 was abolished. Na,K-ATPase activity was not altered in the epithelium of lenses that had been exposed to PP2 alone (Fig. 2). The effect of ET-1 on Na,K-ATPase activity was also abolished when lenses were exposed to ET-1 in the presence of an ET receptor antagonist, PD145065 (10 μM), which antagonizes both ETA and ETB receptors (Fig. 3).
Fig. 1.
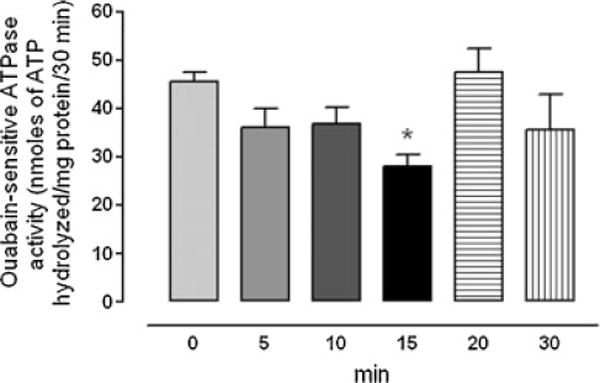
Time course showing changes of Na,K-ATPase activity measured in the epithelium removed from intact porcine lenses following incubation for 5–30 min in the presence of ET-1 (100 nM). The control group (time 0) received no ET-1. The values are the mean ± SE of results from six lenses. *P < 0.05 indicates a significant difference from control.
Fig. 2.

Na,K-ATPase activity measured in the epithelium removed from intact porcine lenses following incubation for 15 min in the presence or absence (control) of ET-1 (100 nM). A different group of lenses was exposed to ET-1 together with the SFK inhibitor PP2 (10 mM) added 45 min prior to ET-1. Another group received PP2 alone. The values are the mean ± SE of results from six lenses. *P < 0.05 indicates a significant difference from control and #P < 0.05 indicates a significant difference from ET-1 alone.
Fig. 3.
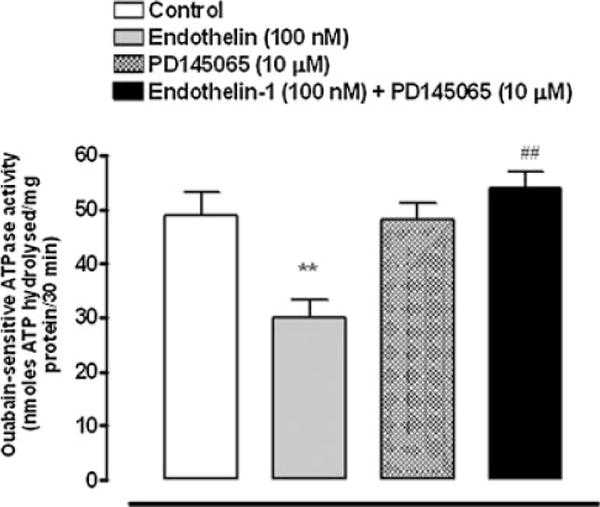
Na,K-ATPase activity measured in the epithelium removed from intact porcine lenses following incubation for 15 min in the presence or absence (control) of ET-1 (100 nM). A different group of lenses was exposed to ET-1 together with the ET receptor antagonist, PD145065 (10 μM) added 30 min prior to ET-1. Another group received PD145065 alone. The values are the mean ± SE of results from 6 to 8 lenses. **P < 0.01 indicates a significant difference from control and # #P < 0.01 indicates a significant difference from ET-1 alone.
Effects on SFKs
The Src family of tyrosine kinases includes nine members. SFK activation was examined by Western blot analysis using two different antibodies, one that detects SFK tyrosine phosphorylation at Y416 and one that detects SFK tyrosine phosphorylation at Y527. Phosphorylation at Y527 represses SFK activity (Yang et al., 2009) and consequently a dense pY527 band signifies an inactive SFK. In epithelium removed from control lenses, a dense pY527-immunoreactive band was detected as 61 kDa along with several more faint bands (Fig. 4A). In epithelium samples obtained from intact lenses that had been exposed to ET-1 (100 nM) for 15 min, the 61 kDa pY527 band density was significantly diminished (Fig. 4B,D). There was no detectable change in density of the minor pY527 bands (data not shown). The reduction in pY527 immunoreactivity points to SFK activation in the epithelium of lenses exposed to ET-1. Phosphorylation at Y416 increases SFK activity and a dense pY416 band signifies an active SFK. A 61 kDa pY416 immunoreactive band was observed in control lenses. Consistent with SFK activation, the epithelium of ET-1-treated lenses displayed a significant increase in density of the 61 kDa pY416 immunoreactive band (Fig. 4C,D). The magnitude of the increase in density of the 61 kDa pY416 immunoreactive band was greatest in lenses exposed to 100 nM ET-1 for 15 min (Fig. 5). No such increase in pY416 band density was observed in epithelium samples from lenses exposed to ET-1 in the presence of PP2 (Fig. 6).
Fig. 4.
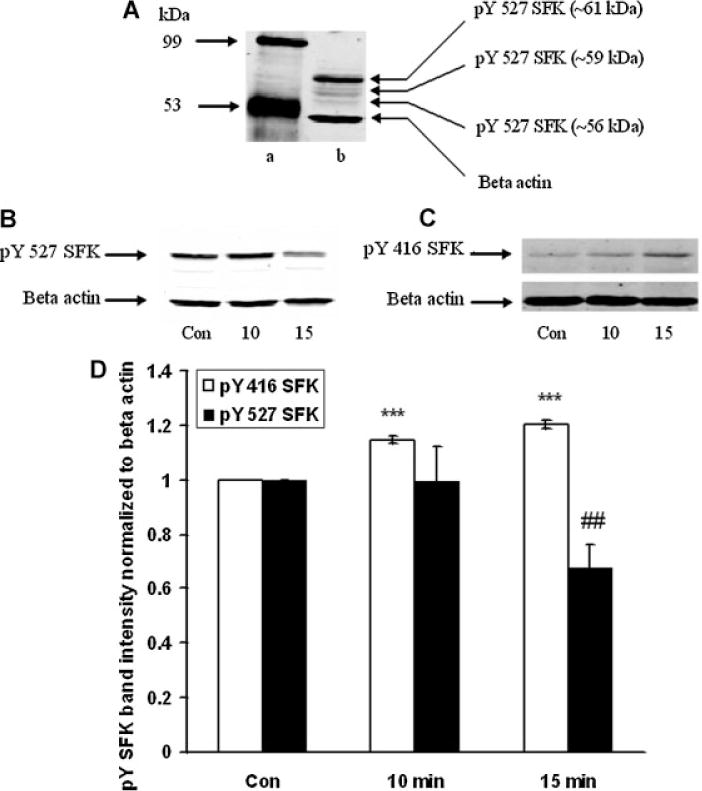
Part A: A Western blot showing pY527 SFK-immunoreactive bands determined in the epithelium of control lenses. The most abundant SFK appeared at a molecular weight ~61 kDa. The blot has been overexposed to reveal the presence of additional, less dense, bands at ~59 and ~56 kDa. Lane a: mol weight standards, and lane b: SFK bands in control lens. Part B: Changes in density of the 61 kDa pY527 SFK-immunoreactive bandin epitheliumremoved from intact lensesthathad been incubated in thepresence ofET-1 (100 nM) for 0(Control), 10or 15 minwhilepart(C) shows changes in density of the 61 kDa pY416 SFK-immunoreactive band. In each case, β-actin served as loading control. Part D: Pooled data on pY527 SFK or pY416 SFK band density relative to β-actin band density. The values are the mean ± SE of results from three independent experiments. * * *P < 0.001 or # #P < 0.01 indicates a significant difference from respective control.
Fig. 5.
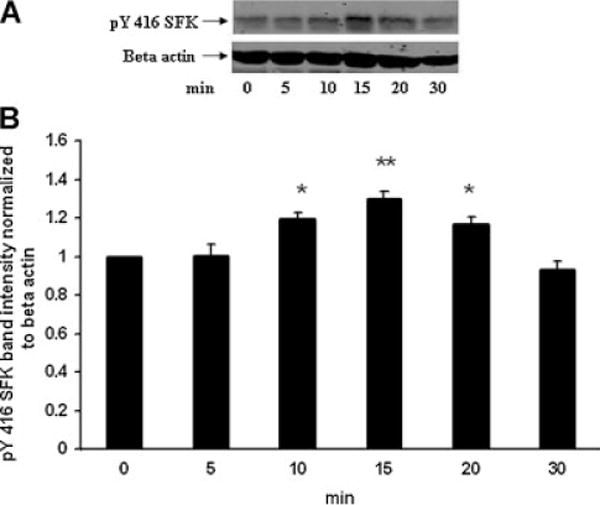
Time course showing changes of SFK phosphorylation detected in the epithelium removed from intact porcine lenses following incubation for 5–30 min in the presence of ET-1 (100 nM). The control group (time 0) received no ET-1. Part A: A Western blot showing pY416 SFK-immunoreactive band density. β-actin served as a loading control. Part B: Pooled data on pY416 SFK band density relative to β-actin band density. The values are the mean ± SE of results from three lenses. *P < 0.05 and **P < 0.01 indicates a significant difference from control.
Fig. 6.
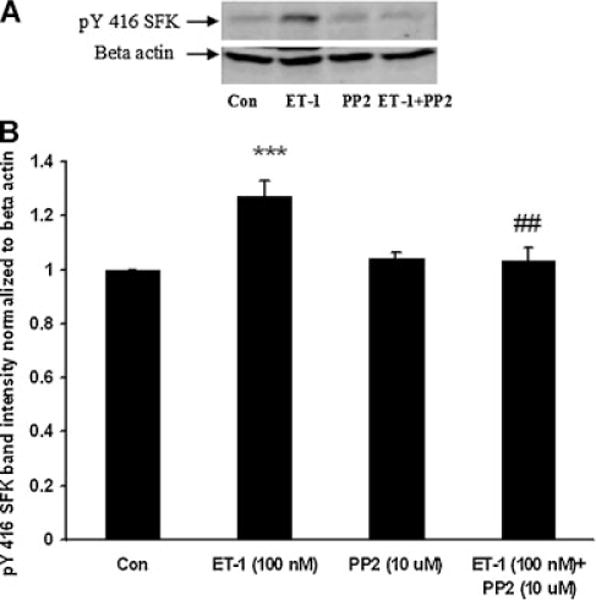
Part A: A Western blot showing pY416 SFK-immunoreactive band density in the epithelium of intact lenses that had been exposed for 15 min to ET-1 (100 nM) in the presence or absence of the SFK inhibitor PP2 (10 μM) added 45 min prior to ET-1. Some lenses received PP2 alone. Control lenses received neither ET-1 nor PP2. β-actin served as a loading control. Part B: Pooled data on pY416 SFK band density relative to β-actin band density. The values are the mean ± SE of results from three independent experiments. ***P < 0.001 indicates a significant difference from the control and ##P < 0.01 indicates a significant difference from ET-1 treatment.
When subjected to electrophoresis, Src, Hck, Fyn, and Yes each migrate similarly to the major 61 kDa pY527 and pY416 bands described above. Altered phosphorylation of one or all these SFKs could potentially have led to the observed density increase of the SFK pY416 band in the epithelium of ET-1-treated lenses. A strategy of immunoprecipitation was used to examine each of these SFKs separately. The epithelium was removed from ET-1-treated lenses, homogenized, subjected to immunoprecipitation with an antibody directed against Src, then probed for pY416. After 15 min, a significant reduction in pY416 band density was observed (Fig. 7). Hck also displayed a reduction in pY416 band density (Fig. 8). In contrast, when immunoprecipitation was carried out with an antibody directed against Fyn, a significant increase in pY416 band density was observed (Fig. 9). Yes displayed no detectable alteration in pY416 band density (Fig. 10). The findings suggest increased Fyn activity but diminished Src and Hck activity.
Fig. 7.
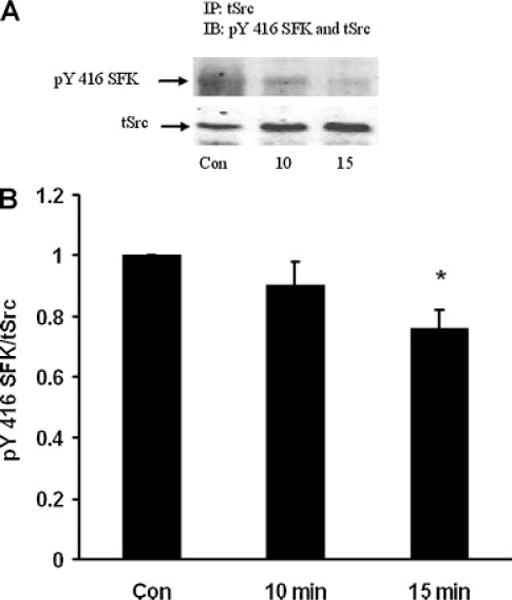
Part A: A Western blot showing pY416 SFK-immunoreactive band density of Src kinase isolated by immunoprecipitation from the epithelium of intact lenses that had been incubated for 0 (Control), 10 or 15 min in the presence of ET-1 (100 nM). As a loading control, total Src protein (tSrc) immunoreactive band density was measured in the same blot. Part B: Pooled data on pY416 SFK band density relative to tSrc band density. The values are the mean ± SE of results from three independent experiments. *P < 0.05 indicates a significant difference from control.
Fig. 8.
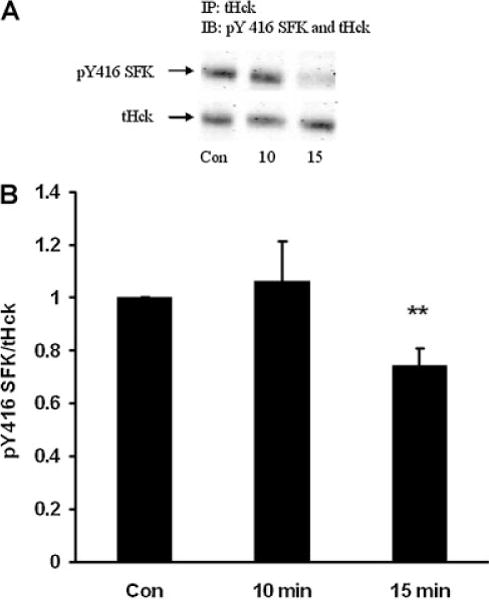
Part A: A Western blot showing pY416 SFK-immunoreactive band density of Hck kinase isolated by immunoprecipitation from the epithelium of intact lenses that had been incubated for 0 (Control), 10 or 15 min in the presence of ET-1 (100 nM). As a loading control, total Hck protein (tHck) immunoreactive band density was measured in the same blot. Part B: Pooled data on pY416 SFK band density relative to tHck band density. The values are the mean ± SE of results from three independent experiments. **P < 0.01 indicates a significant difference from control.
Fig. 9.
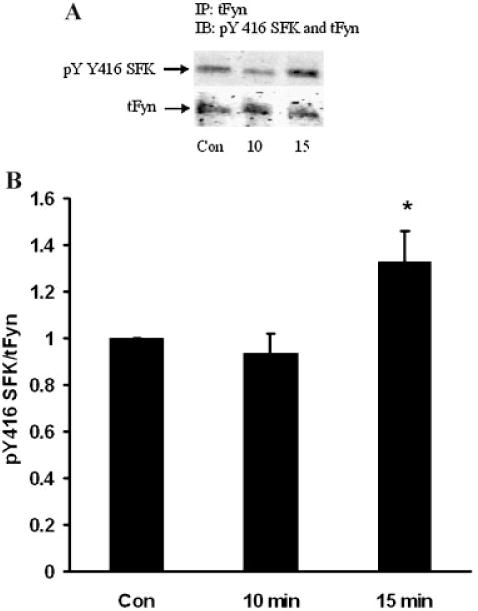
Part A: A Western blot showing pY416 SFK-immunoreactive band density of Fyn kinase isolated by immunoprecipitation from the epithelium of intact lenses that had been incubated for 0 (Control), 10 or 15 min in the presence of ET-1 (100 nM). As a loading control, total Fyn protein (tFyn) immunoreactive band density was measured in the same blot. Part B: Pooled data on pY416 SFK band density relative to tFyn band density. The values are the mean ± SE of results from three independent experiments. *P < 0.05 indicates a significant difference from control.
Fig. 10.
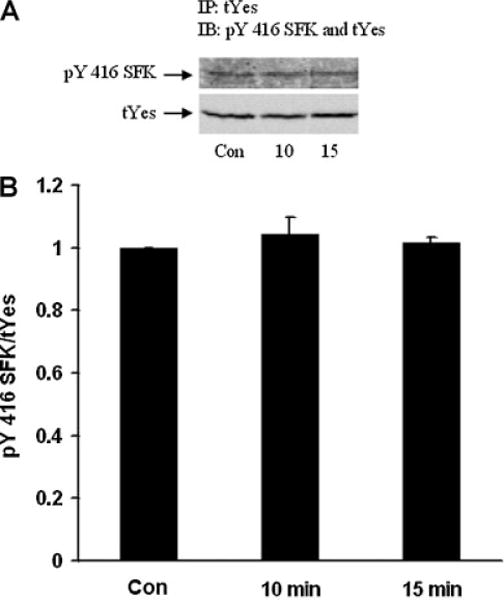
Part A: A Western blot showing pY416 SFK-immunoreactive band density of Yes kinase isolated by immunoprecipitation from the epithelium of intact lenses that had been incubated for 0 (Control), 10 or 15 min in the presence of ET-1 (100 nM). As a loading control, total Yes protein (tYes) immunoreactive band density was measured in the same blot. Part B: Pooled data on pY416 SFK band density relative to tYes band density. The values are the mean ± SE of results from three independent experiments.
Discussion
Exposure of the intact lens to ET-1 caused an intrinsic change of Na,K-ATPase activity in the epithelium. The reduction in Na, K-ATPase activity, which was greatest after 15 min ET-1 treatment, was evident after isolation and homogenization of the epithelium, under conditions where ATP, Na–K concentrations were optimal and no ET-1 was present. The Na,K-ATPase response is consistent with an earlier study that showed ET-1 causes a functional reduction of active Na–K transport (ouabain-sensitive 86Rb uptake) by intact lenses (Okafor and Delamere, 2001). The Na,K-ATPase activity response was abolished by the ETA and ETB receptor antagonist PD145065, as was the previously reported stimulation of ouabain-sensitive 86Rb uptake by intact lenses (Okafor and Delamere, 2001). In that earlier study, the response was abolished by genistein, a nonselective tyrosine kinase inhibitor and it was concluded that ET-1 causes inhibition of lens active Na–K transport by a mechanism that involves activation of ET receptors followed by activation of a tyrosine kinase. Here, we present evidence showing an effect of ET-1 on several members of the Src family of tyrosine kinases. The specific SFK inhibitor PP2 prevented the reduction of Na,K-ATPase activity in ET-1-treated lenses.
The folding state and activity of SFKs depends on tyrosine phosphorylation that occurs primarily at two sites (Yang et al., 2009). SFK activation was evident in the lens after ET-1 exposure. Firstly, there was an increase in Western blot band density of a 61 kDa pY416 SFK-immunoreactive protein in the epithelium of lenses exposed to ET-1. The anti-PY416 Src antibody cross-reacts with SFK members phosphorylated at the active loop tyrosine (PYA-SFK). Secondly, there was a decrease Western blot band density of a 61 kDa pY527 SFK-immunoreactive protein. The anti-pY527 Src antibody cross-reacts with SFK members phosphorylated at the COOH-terminal inhibitory tyrosine (PYT-SFK). In other words, Y527 phosphorylation represses SFK activity. Taken together, the increase of pY416 and decrease of pY527 indicates activation of one or more SFKs having a size ~61 kDa. It is noteworthy that the increase of pY416 band density was greatest after 15 min ET-1 treatment, a time when the greatest reduction in Na,K-ATPase activity in the epithelium was observed.
The part played by SFKs in Na,K-ATPase regulation is not easy to understand. UTP (or ATP) and ET-1 both cause SFK activation but have opposite effects on Na,K-ATPase activity. The SFK inhibitor PP2 is known to prevent the stimulation of Na,K-ATPase activity caused by treating lenses with ATP or UTP, but here we show PP2 also prevents the inhibition of Na,K-ATPase activity caused by treating lenses with ET-1. SFKs are widely expressed and several members of this tyrosine kinase family have been detected in the lens. The multiple pY527 SFK Western blot bands (inactive form) that we observed can likely be attributed to different family members. However, in the epithelium from ET-1-treated lenses the decrease of pY527 band density, indicating SFK activation, was detected only for the major band at 61 kDa. This could signify Src, Fyn, Yes, or Hck activation because these four members of the Src family migrate similarly upon SDS gel electrophoresis. An immunoprecipitation strategy was devised to separately examine Src, Fyn, Yes, and Hck activation. Each of these kinases was immunoprecipitated, and then probed for pY416 since an increase in pY416 band density is more readily detected than a partial decrease in pY527 band density.
Exposing the intact lens to ET-1 caused a significant increase in Fyn pY416 band density in the epithelium consistent with Fyn tyrosine kinase activation. Fyn was the only SFK that displayed activation. pY416 band density for Yes was not detectably altered. ET-1 treatment reduced Src and Hck activity based on diminished Y416 phosphorylation observed for Src and Hck in the epithelium of ET-1-treated lenses. It should be recognized that changes in pY416 and pY527 are an indirect assessment of SFK activation and direct measurements of Fyn, Src, Yes, or Hck activity are not feasible. Moreover, we cannot rule out the possibility that small changes in Y416 phosphorylation of other SFKs went undetected. Nevertheless, the evidence points to selective activation of Fyn in lenses exposed to ET-1. The ability of the SFK inhibitor PP2 to prevent both SFK activation and the decrease of Na,K-ATPase activity in ET-1 treated lenses points to a role for Fyn activation in the Na,K-ATPase inhibition response.
Clearly purinergic agonists and ET-1 cause different patterns of tyrosine kinase activation in the lens epithelium. Based on Y416 phosphorylation, ET-1 appears to activate Fyn, while Src and Hck are inhibited and has no effect on Yes kinase. ATP and UTP activate Src, and to a much lesser extent Fyn. Different Na,K-ATPase responses are associated with the different patterns of SFK activation. The pattern of changes in Fyn and Src activation elicited by ET-1, as evidenced by phosphorylation, fits with an earlier study in which partially purified Fyn was found to inhibit Na,K-ATPase activity in a lens epithelium membrane preparation while Src increased Na,K-ATPase activity (Bozulic et al., 2005). Fyn activation and Src inhibition could both contribute to the Na,K-ATPase inhibition in the epithelium of lenses exposed to ET-1.
ET-1 is best recognized as a peptide vasoconstrictor but it elicits responses in a variety of tissues. It is known to activate Src, Yes, and Fyn in cardiac myocytes (Kovacic et al., 1998) where it may be a step in a hyprertrophic response. ET-1 has been reported to inhibit Na,K-ATPase activity in several tissues including renal tubule and ocular nonpigmented ciliary epithelium (Prasanna et al., 2001; Krishnamoorthy et al., 2003; Liu et al., 2009). In other tissues ET-1 stimulates Na,K-ATPase activity (Gupta et al., 1991; Kawai et al., 1995). Different Na, K-ATPase responses have been thought to reflect differences in ETA versus ETB receptor expression profiles in different cell types. The ET receptor subtype in the lens is not known. The lens appears capable of ET-1 synthesis and can accumulate the peptide from the surrounding medium (Okafor et al., 2002). Release of ET-1 from the lens is tightly controlled (Okafor et al., 2002). The ability of the lens to synthesize, store and release ET-1, coupled with evidence for ET receptor-mediated responses, suggests ET-1 might act in an autocrine manner. At this time, the purpose of such putative ET-1 signaling remains obscure. It could be a way to coordinate function of the epithelium with the bulk of the lens that comprises fibers cells with negligible Na,K-ATPase activity.
Acknowledgments
The authors are thankful to the University of Arizona Meat Science laboratory and Hatfield Quality Meat, Pennsylvania for the supply of porcine eyes. This research was supported by a grant from the National Institute of Health (NIH EY009532).
Contract grant sponsor: National Institute of Health; Contract grant number: NIH EY009532.
Literature Cited
- Bozulic LD, Dean WL, Delamere NA. The influence of protein tyrosine phosphatase-1B on Na,K-ATPase activity in lens. J Cell Physiol. 2004;200:370–376. doi: 10.1002/jcp.20029. [DOI] [PubMed] [Google Scholar]
- Bozulic LD, Dean WL, Delamere NA. The influence of SRC-family tyrosine kinases on Na,K-ATPase activity in lens epithelium. Invest Ophthalmol Vis Sci. 2005;46:618–622. doi: 10.1167/iovs.04-0809. [DOI] [PubMed] [Google Scholar]
- Collison DJ, Duncan G. Regional differences in functional receptor distribution and calcium mobilization in the intact human lens. Invest Ophthalmol Vis Sci. 2001;42:2355–2363. [PubMed] [Google Scholar]
- Delamere NA, Dean WL. Distribution of lens sodium–potassium–adenosine triphosphatase. Invest Ophthalmol Vis Sci. 1993;34:2159–2163. [PubMed] [Google Scholar]
- Delamere NA, Tamiya S. Lens ion transport: From basic concepts to regulation ofNa,K-ATPase activity. Exp Eye Res. 2009;88:140–143. doi: 10.1016/j.exer.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Beialy W, Galal N, Deyama Y, Yoshimura Y, Suzuki K, Tei K, Totsuka Y. Regulation of human and pig renal Na(+),K (+)-ATPase activity by tyrosine phosphorylation of their alpha(1)-subunits. J Membr Biol. 2010;233:119–126. doi: 10.1007/s00232-010-9231-z. [DOI] [PubMed] [Google Scholar]
- Gupta S, Ruderman NB, Cragoe EJ, Jr, Sussman I. Endothelin stimulates Na(+)-K(+)-ATPase activity by a protein kinase C-dependent pathway in rabbit aorta. Am J Physiol Heart Circ Physiol. 1991;261:H38–H45. doi: 10.1152/ajpheart.1991.261.1.H38. [DOI] [PubMed] [Google Scholar]
- Kawai N, McCarron RM, Spatz M. Endothelins stimulate sodium uptake into rat brain capillary endothelial cells through endothelin A-like receptors. Neurosci Lett. 1995;190:85–88. doi: 10.1016/0304-3940(95)11507-s. [DOI] [PubMed] [Google Scholar]
- Kovacic B, Ilic D, Damsky CH, Gardner DG. c-Src activation plays a role in endothelin-dependent hypertrophy of the cardiac myocyte. J Biol Chem. 1998;273:35185–35193. doi: 10.1074/jbc.273.52.35185. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy RR, Prasanna G, Dauphin R, Hulet C, Agarwal N, Yorio T. Regulation of Na,K-ATPase expression by endothelin-1 in transformed human ciliary non-pigmented epithelial (HNPE) cells. J Ocul Pharmacol Ther. 2003;19:465–481. doi: 10.1089/108076803322473024. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yang J, Ren H, He D, Pascua A, Armando MI, Yang C, Zhou L, Felder RA, Jose PA, Zeng C. Inhibitory effect of ETB receptor on Na(+)-K(+) ATPase activity by extracellular Ca(2+) entry and Ca(2+) release from the endoplasmic reticulum in renal proximal tubule cells. Hypertens Res Clin Exp. 2009;32:846–852. doi: 10.1038/hr.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurtz MM, Louis CF. Purinergic receptor-mediated regulation of lens connexin43. Invest Ophthalmol Vis Sci. 2007;48:4177–4186. doi: 10.1167/iovs.06-0496. [DOI] [PubMed] [Google Scholar]
- Luttrell DK, Luttrell LM. Not so strange bedfellows: G-protein-coupled receptors and Src family kinases. Oncogene. 2004;23:7969–7978. doi: 10.1038/sj.onc.1208162. [DOI] [PubMed] [Google Scholar]
- Ma YC, Huang J, Ali S, Lowry W, Huang XY. Src tyrosine kinase is a novel direct effector of G proteins. Cell. 2000;102:635–646. doi: 10.1016/s0092-8674(00)00086-6. [DOI] [PubMed] [Google Scholar]
- Okafor MC, Delamere NA. The inhibitory influence of endothelin on active sodium– potassium transport in porcine lens. Invest Ophthalmol Vis Sci. 2001;42:1018–1023. [PubMed] [Google Scholar]
- Okafor MC, Mukhopadhyay P, Delamere NA. Studies on endothelin release and Na,K transport in porcine lens. Invest Ophthalmol Vis Sci. 2002;43:790–796. [PubMed] [Google Scholar]
- Prasanna G, Dibas A, Hulet C, Yorio T. Inhibition of Na+/K+-ATPase by endothelin-1 in human nonpigmented ciliary epithelial cells. J Pharmacol Exp Ther. 2001;296:966–971. [PubMed] [Google Scholar]
- Sandiford SDE, Green HJ, Ouyang J. Mechanisms underlying increases in rat soleus Na+-K+-ATPase activity by induced contractions. J Appl Physiol. 2005;99:2222–2232. doi: 10.1152/japplphysiol.00577.2005. [DOI] [PubMed] [Google Scholar]
- Shahidullah M, Delamere NA. NO donors inhibit Na,K-ATPase activity by a protein kinase G-dependent mechanism in the nonpigmented ciliary epithelium of the porcine eye. Br J Pharmacol. 2006;148:871–880. doi: 10.1038/sj.bjp.0706795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tamiya S, Okafor MC, Delamere NA. Purinergic agonists stimulate lens Na,K-ATPase-mediated transport via a Src tyrosine kinase-dependent pathway. Am J Physiol. 2007;293:C790–C796. doi: 10.1152/ajpcell.00579.2006. [DOI] [PubMed] [Google Scholar]
- Wallick ET, Schwartz A. Interaction of cardiac glycosides with Na+, K+-ATPase. Methods Enzymol. 1988;156:201–213. doi: 10.1016/0076-6879(88)56022-6. [DOI] [PubMed] [Google Scholar]
- Xie ZJ, Wang YH, Ganjeizadeh M, McGee R, Jr, Askari A. Determination of total (Na++ K+)-ATPase activity of isolated or cultured cells. Anal Biochem. 1989;183:215–219. doi: 10.1016/0003-2697(89)90470-3. [DOI] [PubMed] [Google Scholar]
- Yang S, Banavali NK, Roux BT. Mapping the conformational transition in Src activation by cumulating the information from multiple molecular dynamics trajectories. Proc Natl Acad Sci USA. 2009;106:3776–3781. doi: 10.1073/pnas.0808261106. [DOI] [PMC free article] [PubMed] [Google Scholar]


