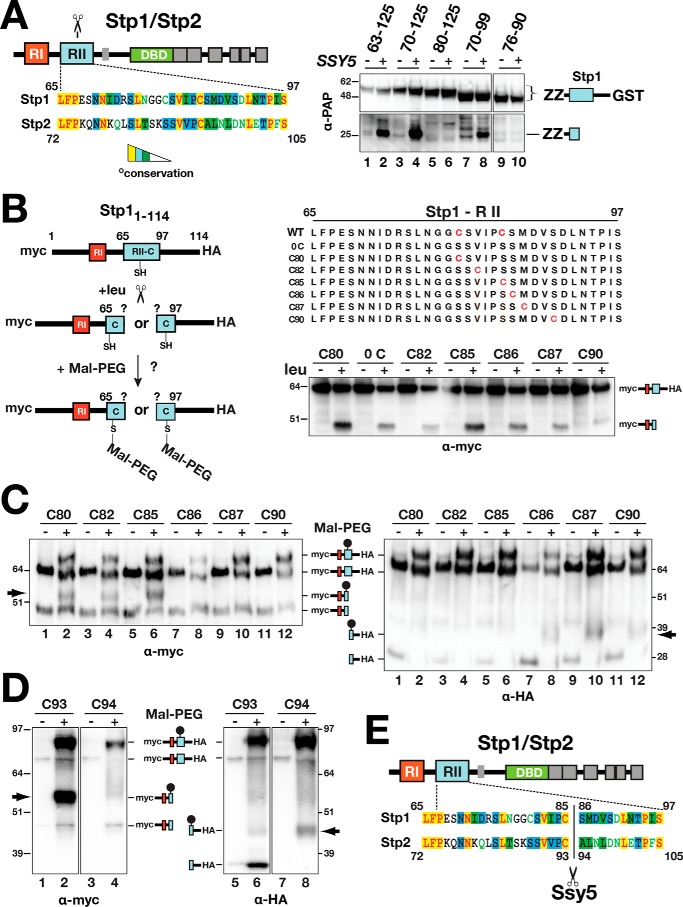
Figure 3.
Determination of the Ssy5 cleavage site in Stp1 and Stp2. A, schematic representation of Stp1 and Stp2 indicating the location of regulatory regions I (RI) and II (RII) and the DNA-binding domains (DBD) (left panel). The Ssy5 cleavage site (scissors) is within region II. Sequence alignment of region II from Stp1 and Stp2 (AlignX, Vector NTI); identical (yellow), conservative (blue), and similar (green) residues are highlighted, and residues with weak (green text) or no similarity (black) are indicated. Strain CAY328 (ssy5Δ) carrying pRS316 (−) or pSH120 (SSY5, +) and plasmids expressing PGAL1-promoted Stp1 fragments, aa 63–125 (pCA221), aa 70–125 (pCA222), aa 80–125 (pCA237), aa 70–99 (pCA246), or aa76–90 (pCA254) in the context of a fusion between a dual immunoglobulin-binding Z domain (ZZ) and GST, was grown overnight in YP with 2% EtOH and subsequently suspended at an OD600 of 1 in YP with 2% galactose for 2 h to induce expression of the Stp1 fragments. Extracts were analyzed by immunoblot using PAP (1:5000), a polyclonal horseradish peroxidase-conjugated immunocomplex that binds the ZZ tag (right panel). The immunoreactive forms of the Stp1 constructs are depicted at their corresponding positions of migration. B, schematic depiction of the maleimide-PEG labeling strategy to map the scissile bonds in Stp1 cleaved by Ssy5 (left panel). WT 13xMYC-STP11–114-ZZ-6xHA and engineered constructs (right panel) containing no cysteine or a single cysteine residue (red) were individually introduced into CAY123 (stp1Δstp2Δ). Extracts, prepared from the strains grown in SD (−leu) and 30 min after induction by leucine (+leu), were analyzed by immunoblotting with α-myc antibodies; the immunoreactive forms of the myc-tagged constructs are depicted at their corresponding positions of migration (right panel). C, Stp1 cleavage. Strain CAY123 (stp1Δstp2Δ) carrying pAM014 (C80), pAM018 (C82), pAM017 (C85), pAM020 (C86), pAM021 (C87), or pAM022 (C90) was grown in SD, and extracts, prepared 30 min after induction by 1.3 mm leucine, were treated with maleimide-PEG as indicated. Depending on the site of cleavage and the localization of the cysteine residue, the maleimide-PEG label will increase the molecular mass of either the N- or C-terminal fragment by 5 kDa (arrows). Immunoblots were developed with α-myc (left panel) or α-HA (right panel) antibodies. The immunoreactive forms of myc-tagged N- and C-terminal HA-tagged constructs are depicted at their corresponding positions of migration. D, Stp2 cleavage. Immunoblot analysis of protein extracts from strain CAY123 (stp1Δstp2Δ) carrying pAM080 (C93) or pAM081 (C94). Cells were grown, and extracts were treated with maleimide-PEG as in C. The immunoreactive forms of myc-tagged N- and C-terminal HA-tagged constructs are depicted at their corresponding positions of migration. E, summary diagram of the scissile bonds in Stp1 (aa 85/86) and Stp2 (93/94) cleaved by Ssy5. Molecular markers (kDa) are indicated at the position of migration (left and right of immunoblots).