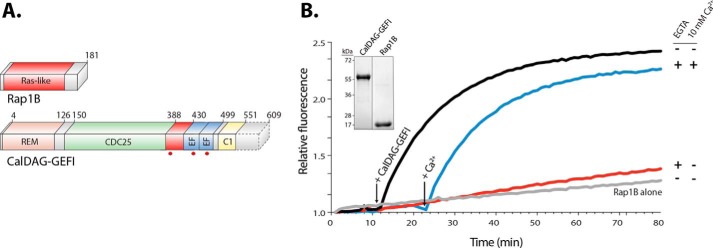
Figure 1.
CalDAG-GEFI requires calcium to activate Rap1B. A, domain architecture. Rap1B is primarily composed of a Ras-like domain (red), whereas CalDAG-GEFI is a multidomain protein consisting of a REM domain (salmon), a catalytic Cdc25 domain (green), a putative autoinhibitory linker (red), two calcium-binding EF hands (blue), and an atypical C1 domain (yellow). CalDAG-GEFI was truncated (dotted lines) at residue 551 for purification. Truncated residues are not conserved and do not impact the capacity of CalDAG-GEFI to activate Rap1B. Substitutions in CalDAG-GEFI used in this work are marked (red circles) below its domain architecture. B, activation of Rap1B by CalDAG-GEFI monitored by the increased fluorescence of BODIPY FL GDP loaded onto Rap1B. Nucleotide (100 nm) and GTPase (1 μm) were incubated in four wells monitored simultaneously (λex/em = 480/520). Select reactions also included 10 mm EGTA as indicated. At 12 min (left arrow), 400 nm CalDAG-GEFI was added to all reactions except the one marked Rap1B alone. Addition of 10 mm free Ca2+ (right arrow) reconstituted the exchange activity. Inset, purified CalDAG-GEFI and Rap1B (2 μg) used in nucleotide exchange reactions; stained gel after SDS-PAGE.