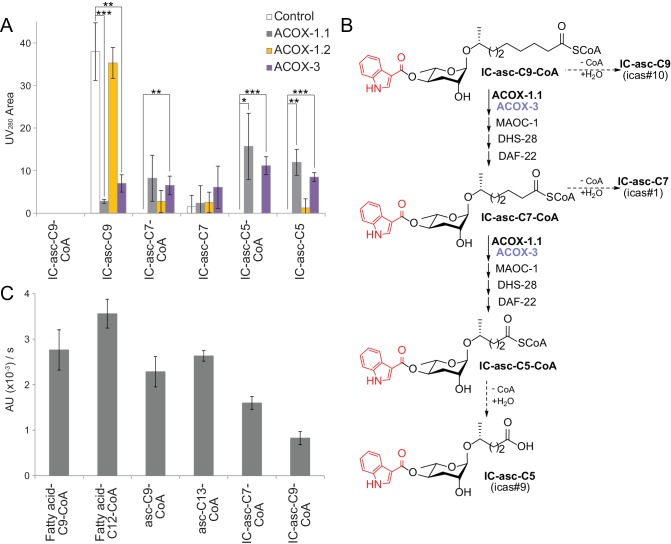
Figure 5. In vitro activity of ACOX-1.1, ACOX-1.2, and ACOX-3.
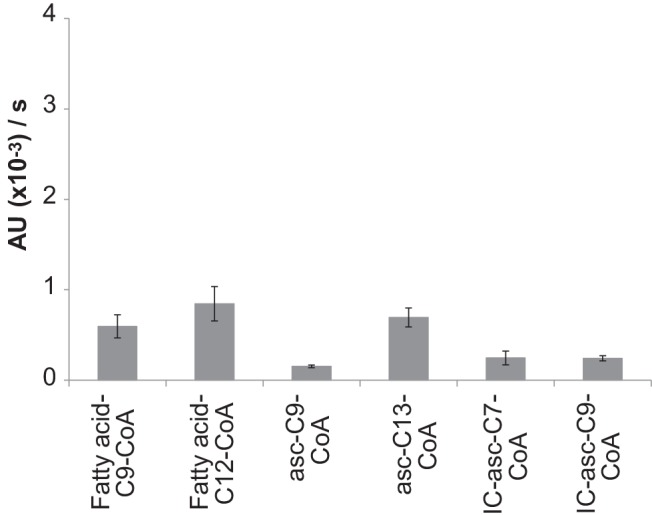
(A) Reaction of IC-asc-C9-CoA with ACOX-1.1, ACOX-1.2, or ACOX-3, in the presence of the additional β-oxidation enzymes, MAOC-1, DHS-28, and DAF-22, as monitored by LC-MS. Chemical structures are shown in (B). Data represent the mean ± SD of three independent experiments. Two-tailed, unpaired t-tests were used to determine statistical significance (*p≤0.05, **p≤0.01, ***p≤0.001). (B) Proposed role for ACOX-1.1 and ACOX-3 in the β-oxidation of IC-asc-C9-CoA to IC-asc-C5-CoA. (C) Activity of ACOX-1.1 in a coupled enzyme assay against the CoA-thioesters of fatty acids (fatty acid-C9-CoA and fatty acid-C12-CoA), ascarosides (asc-C9-CoA and asc-C13-CoA), and IC-modified ascarosides (IC-asc-C7-CoA and IC-asc-C9-CoA). Data represent the mean ± SD of three independent experiments.
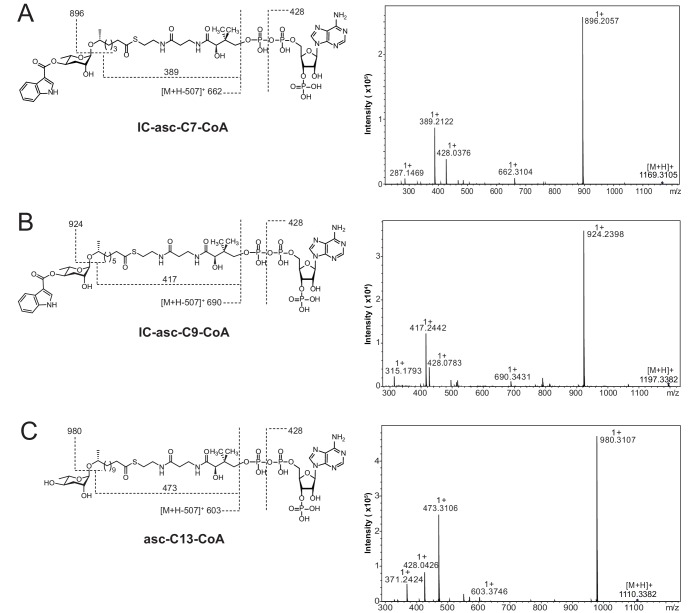
Figure 5—figure supplement 1. High-resolution LC-MS/MS analysis of CoA-thioesters of ascarosides produced chemoenzymatically and used as substrates in Figure 5A,C.
Figure 5—figure supplement 2. Activity of ACOX-3 towards different substrates.