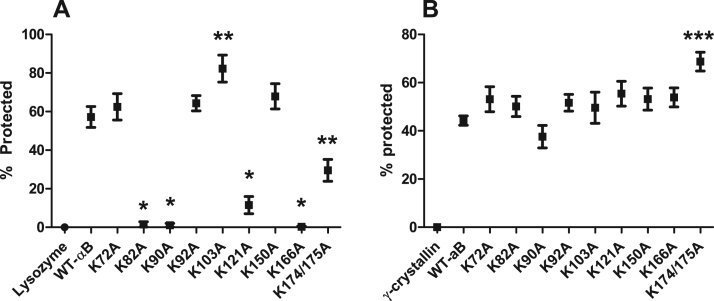
Fig. 2.
Lysine-to-alanine substitution differentially alters chaperone function in αB-crystallin. Wildtype and αB-crystallin substitution mutants were examined for chaperone activity using chemical (A) and thermal (B) aggregation assays. (A) DTT induced lysozyme aggregation was measured for 1 h at 360 nm. Reduction in aggregation of substrate protein alone (lysozyme) by the addition of αB-crystallin was determined to be the percent of protection. (B). Heat induced γ-crystallin aggregation was measured for 1 h at 360 nm. Reduction in γ-crystallin aggregation by the addition of αB-crystallin was determined to be the percent of protection as compared to γ-crystallin alone (γ-crystallin). Statistical changes in rates were determined by ANOVA. p-value > 0.0015 denoted by *, p-values > 0.0001 denoted by ** and p-values > 0.05 denoted by ***.