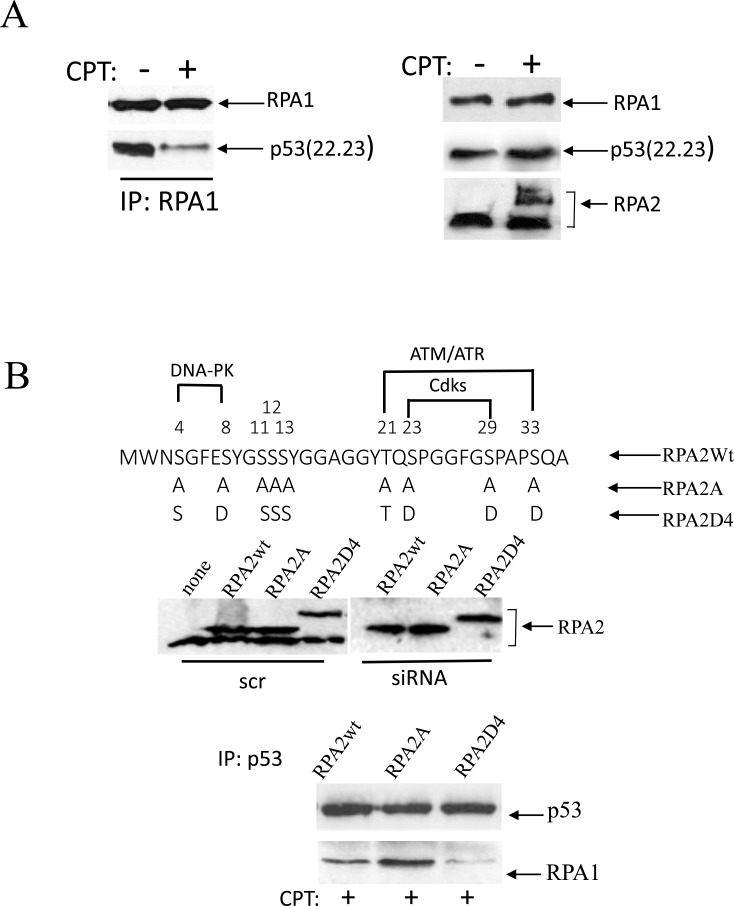
Figure 1. Phosphorylation of RPA in response to replication arrest induced by CPT contributes to the dissociation of the RPA/p53 complex.
(A) Cells were collected for analysis following one-hour treatment with 500 nM CPT. p53-negative H1299 cells were transfected with the transactivation-deficient p53(22.23) mutant. p53 binding was assessed following RPA1 immunoprecipitation. The expression levels of p53(22.23), RPA1 and phosphorylation of RPA2 in untreated cells were used as loading controls. (B) The residues within the N-terminal RPA2 domain reported to be phosphorylated by CDKs, ATR/ATM and DNA-PK were replaced with alanine or glutamic acid, thus producing RPA2A or RPA2D4 mutants that imitate non-phosphorylated or phosphorylated forms of RPA2, respectively. The consensus sites for the kinases are indicated. Expression levels of the recombinant wild type RPA2 and the mutants in A549 cells prior to or after siRNA silencing of endogenous RPA2. The cells with the silenced endogenous RPA2 were treated with 500 nM CPT for one hour. After p53 immunoprecipitation, RPA binding was analyzed on western blots with anti-RPA1 antibody.