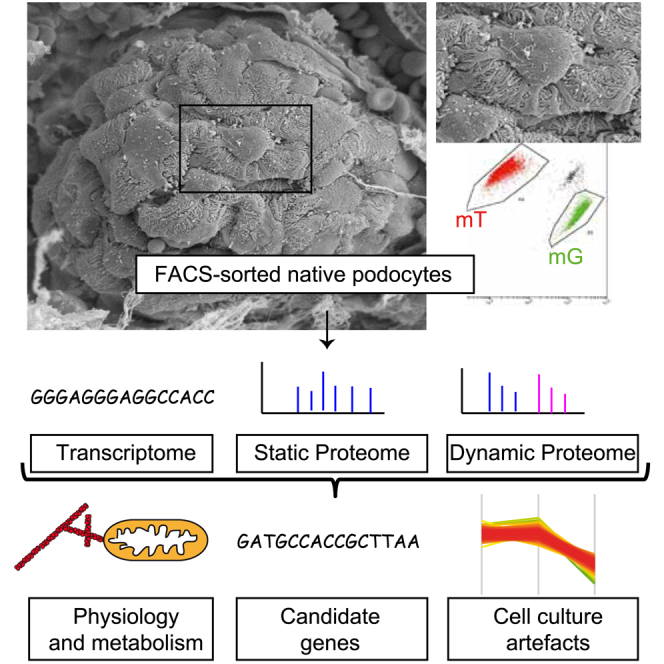
Summary
Damage to and loss of glomerular podocytes has been identified as the culprit lesion in progressive kidney diseases. Here, we combine mass spectrometry-based proteomics with mRNA sequencing, bioinformatics, and hypothesis-driven studies to provide a comprehensive and quantitative map of mammalian podocytes that identifies unanticipated signaling pathways. Comparison of the in vivo datasets with proteomics data from podocyte cell cultures showed a limited value of available cell culture models. Moreover, in vivo stable isotope labeling by amino acids uncovered surprisingly rapid synthesis of mitochondrial proteins under steady-state conditions that was perturbed under autophagy-deficient, disease-susceptible conditions. Integration of acquired omics dimensions suggested FARP1 as a candidate essential for podocyte function, which could be substantiated by genetic analysis in humans and knockdown experiments in zebrafish. This work exemplifies how the integration of multi-omics datasets can identify a framework of cell-type-specific features relevant for organ health and disease.
Keywords: end-stage renal disease, systems biology, proteinuria, focal segmental glomerulosclerosis, pulse SILAC, metabolism, slit diaphragm, hereditary nephrotic syndrome, kinase, proteostasis
Graphical Abstract
Highlights
-
•
Deep proteome and transcriptome analyses of native podocytes unravel druggable targets
-
•
Static and dynamic proteomics uncover features of podocyte identity and proteostasis
-
•
Candidate genes for nephrotic syndrome were predicted based on multi-omic integration
-
•
FARP1 is a previously unreported candidate gene for human proteinuric kidney disease
The podocyte forms the most outer and essential part of the renal filter and restricts the passage of proteins from blood to urine. Rinschen et al. combine deep proteomic and transcriptomic data with protein dynamics from native mouse podocytes to reveal insights into podocyte biology and to identify candidate disease genes.
Introduction
Diseases involving glomeruli, the filtration units of the kidney, are a leading cause of chronic kidney disease (CKD), which affects approximately 15% of all Americans (Meguid El Nahas and Bello, 2005, CDC) and substantially increases cardiovascular events. Despite recent advances in the understanding of glomerular biology, treatment of these disorders has remained extraordinarily challenging in many cases. The podocyte is a postmitotic, neuron-like shaped epithelial cell with limited capacity for self-renewal. Podocytes are essential to maintain a physiological blood-urine barrier (Pavenstädt et al., 2003), and deterioration of podocyte function and subsequent proteinuria are critical accelerators of renal functional decline in disease states. Because of considerable metabolic and mechanical stress, the podocyte needs to maintain its proteome—a prerequisite to maintain its architecture, cytoskeletal integrity, signal transduction, and metabolic function. Interference with any of these functions—for instance, as an inherited gene defect in humans (Boute et al., 2000, Kaplan et al., 2000, Kestilä et al., 1998, Reiser et al., 2005)—leads to proteinuria, podocyte loss, glomerular scarring and, ultimately, chronic kidney disease.
Despite recent progress in the understanding of genetics and signaling of proteinuric kidney disease, the molecular identity of the podocyte is not defined, and it is unclear how its function could be targeted therapeutically. Abundant information regarding podocyte mRNA expression patterns has been gathered recently (Brunskill et al., 2011, Fu et al., 2016, Kann et al., 2015), and integrative genomic studies have enormous potential in classifying human podocyte disease and pathophysiology (Hodgin et al., 2013, Ju et al., 2012, Sampson et al., 2015, Susztak, 2014). However, several studies from different fields found that absolute abundances of mRNA and protein abundances correlate only moderately. Indeed, transcript levels can only explain one- to two-thirds of protein levels, underlining the importance of post-transcriptional control (Liu et al., 2016, Vogel and Marcotte, 2012). In recent years, it became feasible to investigate the entity of proteins, the proteome, at an unprecedented depth (Mann et al., 2013). As a result, proteins can be resolved and quantified relatively and absolutely, culminating in a near-comprehensive mass spectrometry-based expression map of the human proteome (Kim et al., 2014, Wilhelm et al., 2014). In addition, in vivo stable amino acid isotope labeling strategies opened new avenues for the quantification of protein dynamics when used in pulse experiments (Krüger et al., 2008, Savas et al., 2012, Toyama et al., 2013). The aim of this study was to generate a quantitative and integrative map of the podocyte proteome, both static and dynamic, and its transcriptome to gain novel and unbiased insights into podocyte biology. To demonstrate the applications of this “atlas,” we conducted orthogonal hypothesis-driven studies that supported the presence of unanticipated molecular mechanisms maintaining podocyte protein homeostasis and function.
Results
Absolute Quantification of the Native Podocyte Proteome
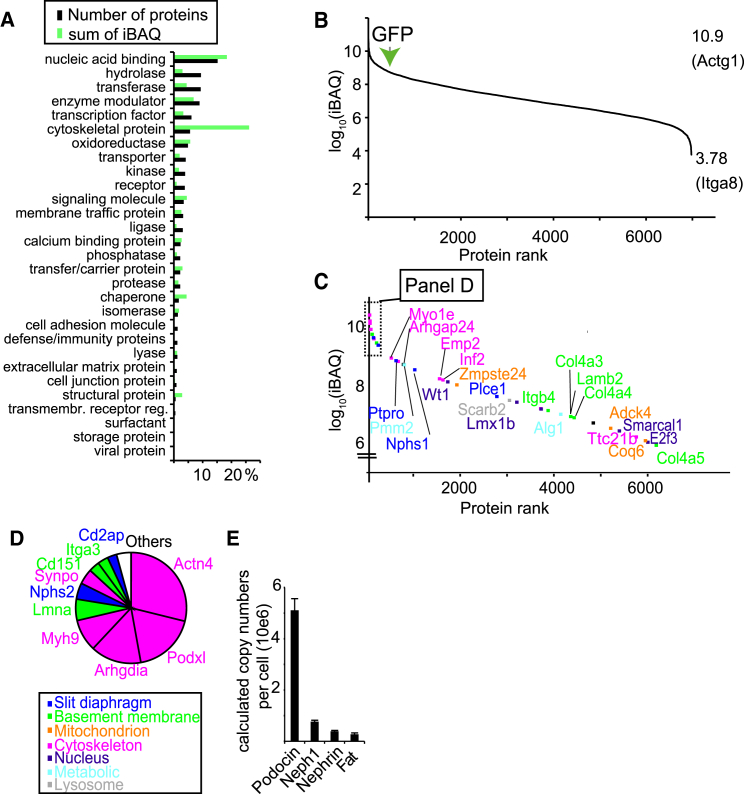
GFP-positive mouse podocytes and tomato-positive non-podocyte glomerular cells were isolated from native glomeruli of an hNPHS2Cre∗mT/mG mouse using fluorescence-activated cell sorting (FACS) (Boerries et al., 2013). Podocytes expressed GFP, whereas all other viable glomerular non-podocyte cells expressed tomato red fluorescent protein (Figure 1A). A work flow for “deep mapping” of transcriptome, proteome, and proteome dynamics was applied to both podocytes and non-podocytes (Kulak et al., 2014). Quality control of the dataset demonstrated a clear separation between both samples (Figure S1). This approach identified, in total, more than 9,000 different proteins (Tables S1 and S2). We performed absolute quantification of 6,979 proteins using the intensity-based absolute quantification (iBAQ) approach (Table S2). This proteomics parameter is relative to copy numbers in a given sample (Kohli et al., 2014, Schwanhäusser et al., 2011). Cytoskeletal proteins were highly represented in the dataset (Figure 1A). The dynamic range of the podocyte proteome copy numbers comprises seven orders of magnitude (Figure 1B). We mapped genes associated with focal segmental glomerular sclerosis or hereditary nephrotic syndrome on this dataset (Figure 1C; top 10 genes in Figure 1D; Bierzynska et al., 2015). These “disease-associated genes,” although only comprising 35 of 7,000 (0.5%) absolutely quantified proteins, contributed approximately 4% of the podocyte protein mass, with cytoskeletal proteins like Actn4 and Arhgdia contributing most to this amount (Figure 1D). Several other known podocyte genes (e.g., Trpc6) were identified but could not be quantified because of their low abundance. To estimate the absolute protein quantity based on the data and determine protein concentrations or copy numbers per cell, we applied a recently developed algorithm on our dataset (“proteomic ruler”) (Wiśniewski et al., 2014). This provides a starting point to determine the stoichiometries of known protein complexes; for example, the prominent transmembrane slit diaphragm complex within native mouse podocytes, which comprises podocin-Neph1-Nephrin-Fat1 with a copy number ratio of ∼10:2:1:0.5 (Figure 1E), indicating that podocin is by far the most abundant slit diaphragm protein within podocytes (Grahammer et al., 2013).
Figure 1.
Absolute Quantification of the Native Podocyte Proteome
(A) Panther term analysis of expressed genes and absolute copy numbers (intensity-based absolute quantification [iBAQ]). iBAQ values corresponding to the number of protein copies (green) and the number of different proteins (black) are plotted.
(B) Overview of the dynamic range of the podocyte proteome absolutely quantified using iBAQ.
(C) Expression of proteins encoded by genes causing hereditary nephrotic syndrome and focal segmental glomerulosclerosis in podocytes according to Bierzynska et al. 2015).
(D) Abundance of the 10 most abundant proteins.
(E) Application of the proteomic ruler to detect protein copy numbers of transmembrane proteins of the slit diaphragm complex (Wiśniewski et al., 2014). Calculated numbers of protein copies per cell are depicted.
Integration of Deep Transcriptomic and Proteomics Data
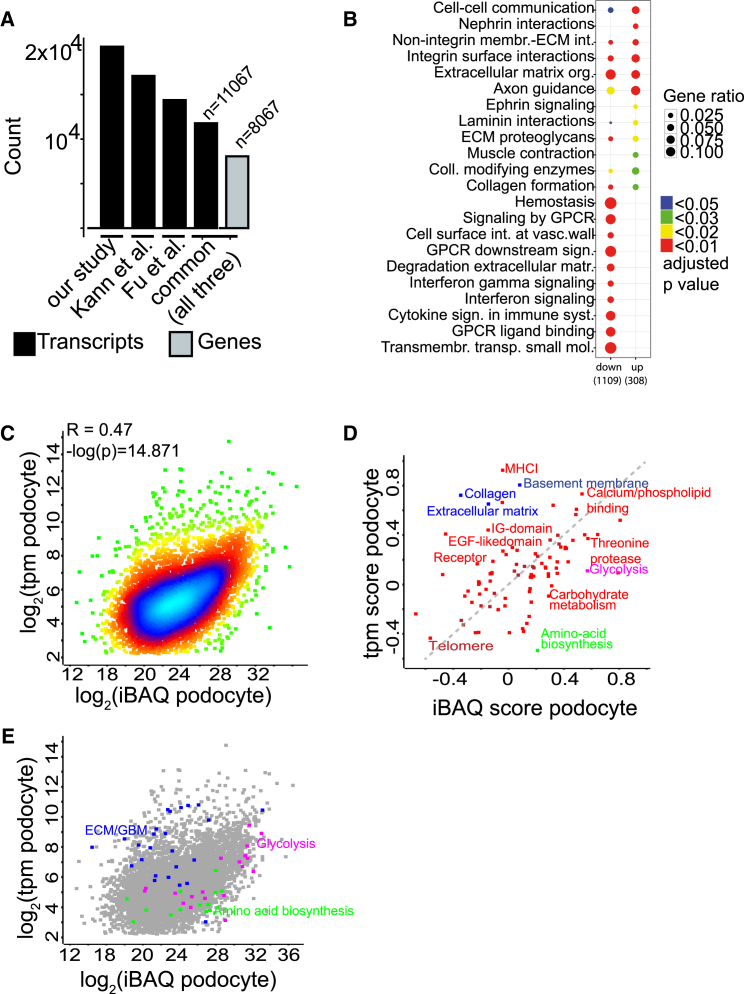
Protein abundance is determined by the rate of transcription but also a result of protein stability and posttranslational processing. To identify the determinants of podocyte protein copy number abundance, we performed comparison with a transcriptomic copy number analysis performed by mRNA sequencing. Our dataset was similar to previously published mRNA sequencing (mRNA-seq) analyses but demonstrated a greater depth upon analysis with the same parameters (Figure 2A; Table S3; Figure S2). Enrichment analyses demonstrated overrepresentation of several known pathways enriched on mRNA levels, among these extracellular matrix (ECM) synthesis and nephrin interactions (Figure 2B; Figures S2E–S2I; Brunskill et al., 2011). To unravel the correlation of transcriptomic data with proteomic data as a whole, iBAQ data were correlated with the transcriptomic (tpm) values, but the correlation was rather weak (Figure 2C). The correlation was stronger for podocytes compared with non-podocytes (Figure 2D). We checked which proteins were specifically stabilized or destabilized posttranscriptionally using 2D enrichment (Cox and Mann, 2012). This algorithm can be used to visualize statistically significant distributions of protein annotations in a 2D space between different “omics” datasets (Cox and Mann, 2012). The analysis revealed that ECM protein expression was decreased as expected by the respective mRNA expression, which would be expected because of loss of podocyte-produced ECM proteins excreted as part of the glomerular basement membrane assembly (Figure 2E, blue). Proteins involved in controlling metabolism were largely stabilized (Figure 2E, magenta, green). These proteins included glycolysis gene products and proteins involved in amino acid biosynthesis.
Figure 2.
Integration of Deep Transcriptomic and Proteomics Data
(A) Comparison of this and other recent transcriptomic (tpm) studies using mRNA-seq. All datasets were processed with the same bioinformatics pipeline as indicated in the Experimental Procedures.
(B) Reactome analysis of transcripts enriched in podocytes (up) and de-enriched in podocytes (down).
(C) Scatterplot demonstrating correlation between proteomic (iBAQ) and (tpm) copy numbers in podocytes (Pearson’s R = 0.47, log(p) = 14.8). The density of individual proteins is color-coded (blue, high density; green, low density).
(D) 2D Uniprot keyword analysis of podocyte transcript and protein copy numbers identifies significantly changed Uniprot keywords with an especially high increase in either omic dataset (permutation-based FDR < 0.05). The dashed gray line (y = x) indicates annotations equally represented in both omic datasets.
(E) Distribution of individual Uniprot keyword members in the original scatterplot (analog to C).
Determination of Podocyte-Enriched Proteins
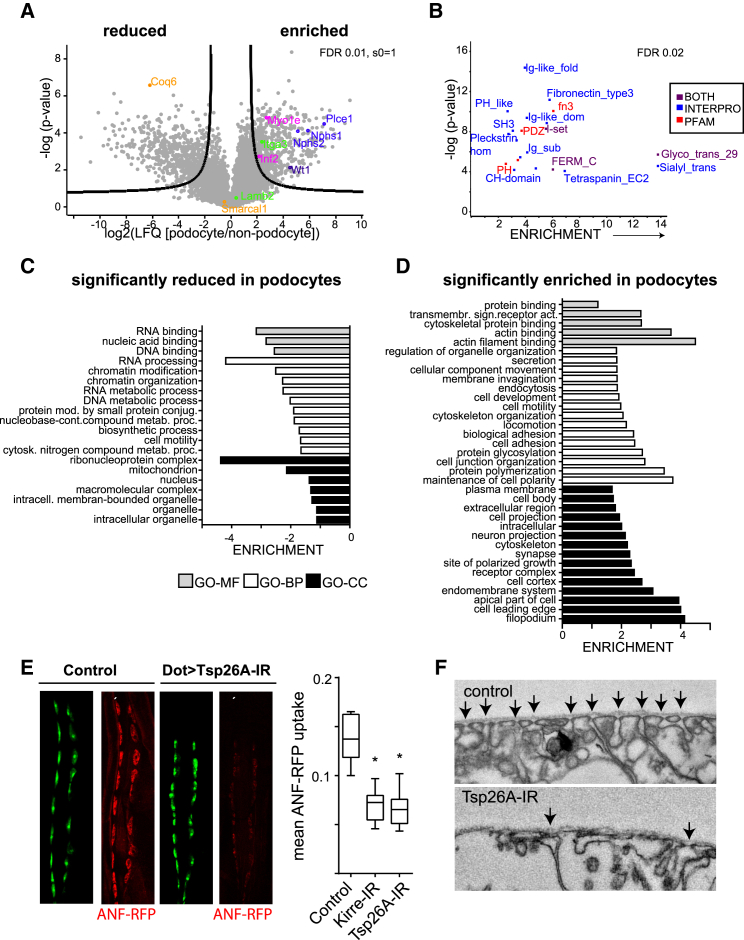
Most of the genes mutated in genetic forms of proteinuria or nephrotic syndrome encode for podocyte-enriched genes. Therefore, we next analyzed whether we could expand the list of known podocyte-enriched proteins compared with other glomerular cells. Using stringent statistical cutoff values for quantification, we found 551 podocyte-enriched proteins (Figure 3A; Table S2); among these were many gene products currently known to be associated with hereditary nephrotic syndrome (Sadowski et al., 2015). Figures S3 and S4A depict standardized stainings of 190 proteins that have a “medium” or “high” staining intensity in the human protein atlas (Uhlén et al., 2015). A systematic Medline search of these 190 proteins revealed that the majority were not explicitly linked to podocytes (Table S4). We performed statistical enrichment for overrepresentation of protein domains in all 551 proteins enriched in podocytes (Figure 3B). Significantly enriched protein domains were Fn3 and immunoglobulin G (IgG)-like domains (e.g., in Nephrin), PDZ domains (e.g., in ezrin and Par-3), i-set domains (e.g., in nephrin and VCAM/NCAM), CH domain (e.g., in actinin-4), and FERM domain proteins (e.g., in ezrin) or tetraspanins (e.g., CD151). On a global level, podocyte-specific proteins were significantly reduced for mitochondria as well as RNA and DNA binding proteins (Figure 3C) and enriched for signaling receptors, cytoskeleton-associated proteins, GTPase-associated proteins, membrane proteins, and (phospho)lipid-modifying proteins (Figure 3D).
Figure 3.
Determination of Podocyte-Enriched Proteins Uncovers Druggable Targets and Candidate Genes for Podocyte Function
(A) Volcano plot of protein quantification of podocytes over non-podocytes. The logarithmic fold change of label-free quantification intensities (LFQs) is plotted versus the negative decadic logarithm of the p value. 551 proteins pass the criteria for significant podocyte enrichment (significance analysis of microarrays [SAM] analysis, s0 = 1, FDR = 0.01).
(B) Enrichment analysis of protein domains in podocytes. Protein domains (PFAM and INTERPO annotation) were tested for enrichment within the podocyte-enriched proteins over the proteins not significantly enriched. –log(p value) of every significantly enriched protein domain is plotted against fold enrichment. Fisher’s exact test, FDR < 0.02.
(C) GO terms overrepresented in the 551 podocyte-enriched proteins compared with non-podocyte specific proteins. Fold enrichment of GO terms representing molecular function (GOMF, gray bars), cellular component (GOCC, black bars), and biological process (GOBP, white bars). All GO terms are statistically enriched in the dataset compared with non-podocyte proteins. Fishers exact test, FDR < 0.05.
(D) Significantly de-enriched GO terms in the 551 podocyte-specific proteins (the same statistical criteria and color code as in C).
(E) CLSM micrographs of pericardial nephrocytes at second larval stage. RNAi-mediated knockdown of Tsp26A significantly reduced ANF-RFP (red) uptake, suggesting that Tsp26A is required for pericardial nephrocyte (green) function. The ANF-RFP uptake is significantly reduced by the knockdown of Tsp26A (p value < 0.05)
(F) Ultrastructural alteration of Tsp26A knockdown nephrocytes. Arrows indicate nephrocyte cell contacts.
To demonstrate that this resource may be used to find essential podocyte proteins, we analyzed the effect of knockdown of one candidate podocyte-enriched gene in Drosophila nephrocytes, an established model of podocyte function (Weavers et al., 2009, Zhang et al., 2013). We knocked down Tsp26A, the Drosophila homolog of TSP5 and TSP15, two newly identified podocyte-enriched proteins that are representatives of the protein class of tetraspanins (Figure 3D). This resulted in significantly decreased ANF-RFP uptake, indicating loss of filtration function (Figure 3E). We also found overt alterations in nephrocyte ultrastructure (Figure 3F), proving the importance of the tetraspanin protein class for nephrocyte function.
Podocyte protein homeostasis largely depends on signaling mediated via posttranslational modifications, such as phosphorylation, ubiquitylation, acetylation, and proteolytic processing (New et al., 2014). Pinpointing podocyte-specific signaling molecules could help us to understand podocyte physiology and target podocyte signaling pharmacologically or genetically. Table 1 summarizes unanticipated observations of signaling molecules enriched in mouse podocytes; among these were several druggable molecules. (For a full list of podocyte enriched kinases, phosphatases, ubiquitin ligases, proteases, signaling receptors, actin binding proteins and transcription factors, see Table S5). We also mapped the 551 podocyte-enriched proteins against known protein interaction databases to obtain a network and central nodes among these podocyte-enriched proteins (Cerami et al., 2010). The proteins RhoA, Actin, and Grb and the nephrin-regulating kinase Src had most edges within these networks (Figure S4B); all four have reported relevance for podocyte biology (Harita et al., 2008, New et al., 2014, Schmieder et al., 2004), suggesting a central role for these proteins.
Table 1.
Examples of Podocyte-Enriched Kinases, Transcription Factors, and Potentially Druggable Targets
| Gene Symbol | Uniprot ID | Log2(Ratio), (−log(p)) | Name | Comment | References |
|---|---|---|---|---|---|
| Csnk1g3 | Q8C4X2 | 2.14 (2.84) | casein kinase 1 gamma 3 | an acidophilic S/T protein kinase that is a candidate for abundant acidophilic phosphorylation on slit diaphragm proteins (Nephrin, Trpc6, and Cd2ap) | Rinschen et al., 2014, Rinschen et al., 2015b |
| Nek1 | B7ZWK0 | 3.43 (3.61) | serine/threonine protein kinase Nek1 | a protein kinase (STY) responsible for ciliogenesis | Shalom et al., 2008 |
| Mst4 | Q99JT2; | 2.89 (4.70) | serine/threonine protein kinase MST4 | members of the canonical hippo pathway, linked to mechanotransduction and apoptosis, druggable | Schwartzman et al., 2015, Rinschen et al., 2017 |
| Lats2 | Q7TSJ6 | 3.25 (2.37) | serine/threonine protein kinase LATS2 | ||
| Tead1 | P30051 | 3.70 [1.83] | transcriptional enhancer factor TEF-1 | ||
| Dach1 | Q9QYB2 | 4.63 (4.22) | dachshund homolog 1 | a transcription factor strongly enriched in podocytes (comparable with WT1), GWAS demonstrate association with GFR | Köttgen et al., 2010 |
| Cblb | B9EKI5;Q3TTA7 | 4.10 (2.23) | E3 ubiquitin-protein ligase CBL-B | strongest enriched ubiquitin ligase in podocytes, localizes in immune cells and mediates the expression of EGF receptors in a protein complex with the recently discovered podocin-associated ubiquitin ligase Ubr4, potentially druggable | Rinschen et al., 2016a, Tong et al., 2014, Wirnsberger et al., 2016 |
| Npr3 | P70180 | 4.89 (1.89) | atrial natriuretic peptide receptor 3 | mediates ANP signaling via cGMP, confirmation of in vitro and in vivo studies | Rinschen et al., 2016b, Staffel et al., 2016 |
| Npr1 | P18293 | 2.38 (3.70) | atrial natriuretic peptide receptor 1 | ||
| Pth1r | P41593 | 6.19 (2.78) | PTH receptor | mediates Pth signaling via cAMP, confirmation of in vitro studies | Endlich and Endlich, 2002 |
| Ifngr1 | P15261 | 3.85 (1.86) | interferon gamma receptor 1 | confirmation of in vitro and in vivo study, druggable | Gurkan et al., 2013 |
| Mertk | Q60805 | 4.83 (4.36) | tyrosine protein kinase Mer | most enriched tyrosine kinase family, involved in cancer and development, druggable | Lee-Sherick et al., 2015, Zhang et al., 2014, Fleuren et al., 2014 |
| Tyro3 | P55144 | 4.30 (3.76) | tyrosine protein kinase receptor TYRO3 | ||
| Axl | Q6PE80 | 4.02 (3.32) | Tyrosine-protein kinase receptor UFO |
Log2(Ratio) is defined as log2(LFQ in podocytes)/(LFQ in non-podocytes) and is a measurement of podocyte enrichment. Table S5 gives an overview of each class of the annotated signaling molecules. GWAS, genome-wide association study; EGF, epidermal growth factor; cAMP, cyclic AMP; GFR, glomerular filtration rate; cGMP, cyclic GMP; PTH, parathyroid hormone; atrial natriuretic peptide (ANP).
Comparison of the Deep Native Podocyte Proteome with the Cultured Podocyte Proteome
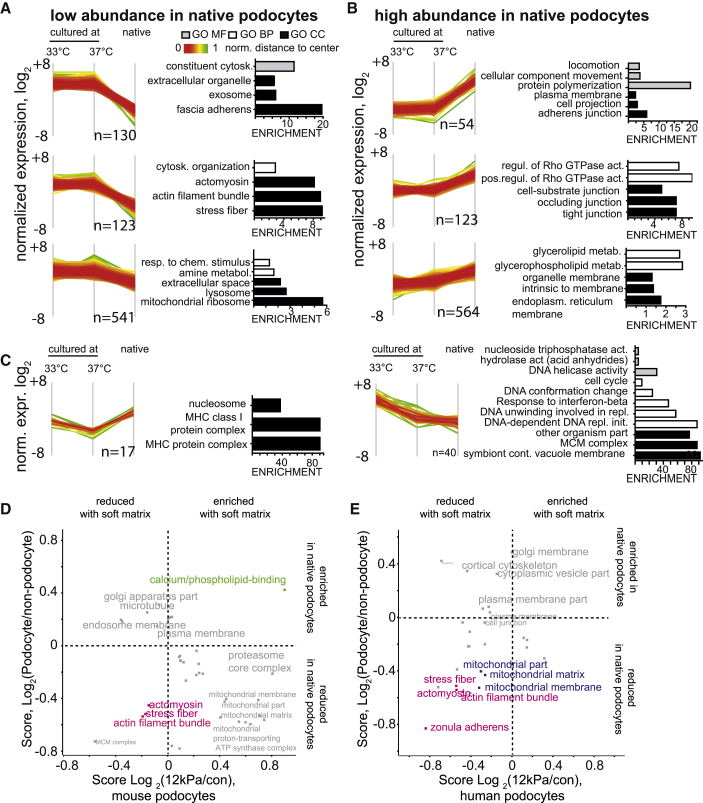
We asked whether this unique obtained podocyte proteome resource would help us to investigate the strengths and weaknesses of in vitro immortalized podocyte cell cultures, a commonly used tool in podocyte research. To this end, we compared the dataset with a similar, previously published deep quantitative proteomic mapping of a mouse podocyte cell line (Rinschen et al., 2016b). We could identify 6,193 proteins in both samples, representing a significant overlap between both samples (Figure S4C). Clustering analysis of normalized expression values revealed significant differences between native podocytes and the in vitro cell line irrespective of the differentiation status (33°C = undifferentiated or 37°C = differentiated; Figure S4D). Clustering analysis revealed eight major clusters. Three clusters contained proteins with high abundance in cell lines compared with the native podocytes and were enriched for proteins related to the actin cytoskeleton, including stress fibers and focal adhesion proteins (Figure 4A). Three clusters contained proteins with low abundance in cell lines compared with the native podocytes (Figure 4B). These clusters were related to phospholipid metabolism and tight junctions. Two clusters showed differences between 33°C and 37°C and native cells; these were, as expected (Rinschen et al., 2016b), related to the cell cycle (Figure 4C). Moreover, disease-causing proteins such as nephrin and podocin or podocyte markers such as WT1 were not at all or only very weakly expressed in podocytes in culture compared with the in vivo dataset.
Figure 4.
Comparison of the Deep Native (Isolated from Living Mice) and Cultured Podocyte Proteome Enables Functional Analysis of Cell Culture-Induced Proteome Artefacts
(A) Protein clusters were defined based on normalized intensities as depicted in Figure S4C–D. Shown are clusters that have a higher intensity in cultured podocytes compared with the native podocyte proteome. Distance to the mean is color-coded in each cluster. GO terms significantly overrepresented in each cluster are depicted (Fishers exact test, p values corrected with FDR < 0.05).
(B) Three clusters have a lower intensity (and abundance) in the podocyte proteome compared with the cultured podocyte proteome. GO terms significantly overrepresented in each cluster are depicted.
(C) Two clusters have different intensities in undifferentiated and differentiated podocytes.
(D) Mouse podocytes were seeded on matrices with 12 kPa, and the proteome was compared with control cell culture dishes. See Figure S5 for details regarding the dataset. The 2D GO enrichment score of fold change on soft matrix (log2 12 kPa/control) cell culture is plotted against the score for podocyte-enrichment (log2(podocyte/non-podocyte)) (Figure 3). The data demonstrate a significant decrease of stress fiber proteins under both conditions (soft versus stiff matrix and native podocyte versus non-podocyte). Significantly changed terms are plotted (FDR < 0.05)
(E) Human podocytes were seeded on matrices with 12 kPa, and the proteome was compared with control cell culture dishes. See Figure S5 for details regarding the dataset.
This comparison of podocyte proteomes from cell culture and from native tissue suggested that podocyte cell cultures have an increased abundance of stress fiber-associated proteins and focal adhesions. We postulated that some of these differences may have been a cell culture artefact because of the mechanical properties of the plastic dish on which these cells were cultivated. As a proof of principle, we cultured podocytes on soft matrix with an elastic modulus of 12 kPa and compared their proteome with podocytes grown in plastic dishes (in the range of gigapascals). This elastic modulus (“stiffness”) of 12 kPa is similar to that of a skeletal muscle cell, a mechanically challenged cell (Gilbert et al., 2010, Janmey and Miller, 2011). Using quantitative proteomics, we found vast differences in the podocyte proteome depending on the matrix on which they were grown (Figures S5A and S5B). On a global level, actin filament proteins and stress fiber proteins were significantly depleted by culturing podocytes on the “soft” matrix, which made them, in this regard, “more similar” to the native podocyte (based on the log2 ratio of podocyte/non-podocyte protein expression) (Figure 4D). A similar picture arose when human podocytes were cultured on soft matrices. The changes in the proteome were larger (Figure S5), and the analysis confirmed again the loss of stress fiber protein expression on the soft matrices (Figure 4E; p and false discovery rate (FDR) < 0.05 in a Fisher’s exact test).
Analysis of Podocyte Proteome Dynamics and Integration with Deep Proteomics Mapping
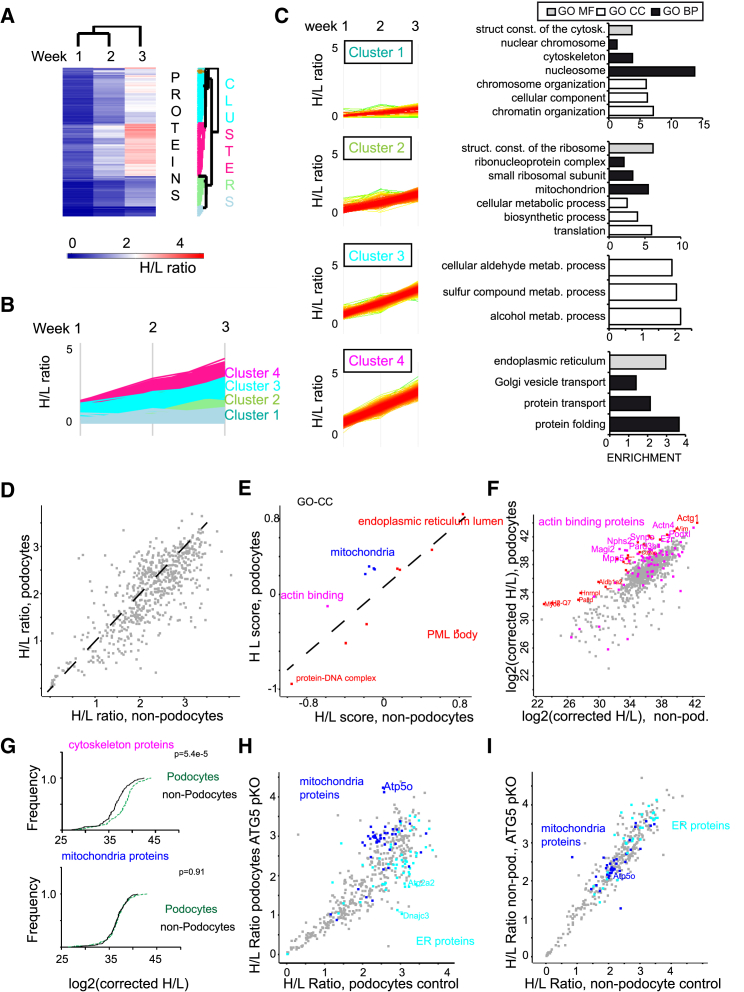
To conclude, our data quantitatively describe expression levels in the podocyte proteome. However, these data did not yet cover an important, rather unexploited component of global proteostasis regulation in vivo: the dynamic synthesis and turnover of proteins. To approach this, we used a mouse model of in vivo stable isotope labeling (Table S6). To this end, adult mice (10 weeks of age) were fed for 1, 2, and 3 weeks with a diet exclusively containing heavy isotope-labeled lysine. The incorporation of this essential amino acid should correlate with the dynamic synthesis rate (although these data do not strictly correlate with a protein “half-life” because of intracellular amino acid recycling (Krüger et al., 2008)). Still, proteins synthesized rapidly should exhibit a high heavy amino acid incorporation rate (and a high heavy/light [H/L] ratio), whereas stable, “long-lived” proteins with slow translation should have a lower incorporation rate (Savas et al., 2012). To see which proteins incorporate isotopes at a similar speed, we performed hierarchical clustering of H/L ratios over the labeling time course. This analysis revealed four major clusters of proteins (Figures 5A and 5B). Gene ontology (GO) term analysis of these four clusters revealed that endoplasmic reticulum (ER)-associated proteins (cluster 4) were particular “dynamic” compared with the nuclear proteins, in particular histones (cluster 1), which only showed minimal increases in the H/L ratio (Figure 5C), consistent with previous studies (Toyama et al., 2013). There was almost no correlation between iBAQ values and H/L ratios (correlation coefficient [R2] = −0.01) (Fig. S6A).
Figure 5.
Integration of Podocyte Proteome Dynamics and Deep Proteomics Mapping Demonstrate Proteostatic Podocyte Features
(A) Adult mice were fed a diet in which lysine was substituted with stable isotope-labeled 13C6 lysine for the indicated times, resulting in a gradual substitution of the endogenous lysine 12C6 lysine (light lysine) with 13C6 lysine (heavy lysine). Mean H/L ratios of more than 4 animals are depicted.
(B) Hierarchical clustering (maximum distance) of mean protein H/L ratios was performed, and four major row clusters were defined.
(C) GO enrichment of GOMF (black), GOCC (gray), and GOBP (white) terms (Fishers exact test, corrected FDR < 0.05) in each of the 4 major clusters was performed.
(D) Scatterplot of H/L ratios between podocytes and non-podocyte cells demonstrates that podocytes have a generally slower incorporation of stable isotope-labeled lysine.
(E) 2D GO enrichment analysis of H/L ratios of significantly regulated Uniprot keywords of podocytes and non-podocyte cells (FDR < 0.05).
(F) Scatterplot of corrected H/L ratios between podocytes and non-podocytes.
(G) Cumulative histogram of corrected H/L ratios for cytoskeletal and mitochondrial proteins. When H/L ratios are corrected with absolute protein abundance (Dotproduct, see Experimental Procedures for details), mitochondrial proteins have a similar corrected ratio compared with all other proteins.
(H) H/L ratios in podocytes from control mice plotted against H/L ratios in podocytes in Nphs2.Cre:Atg5 fl/fl (ATG5ko) mice. ATP5o is a subunit of the mitochondrial ATP synthase.
(I) H/L ratios in non-podocytes from control mice plotted against H/L ratios in non-podocytes of (ATG5ko) mice.
When comparing podocyte H/L ratios with those of co-sorted non-podocyte cells, we observed that podocyte proteins incorporated more slowly than the parallel sorted non-podocyte cells (Figure 5D). 2D GO analysis revealed that cytoskeletal and mitochondrial proteins had a particularly high protein turnover in podocytes compared with non-podocyte cells (Figure 5E). We also analyzed an intact whole kidney protein sample from an independent parallel study (Figures S6B and S6C), confirming that mitochondrial proteins were incorporated relatively faster in podocytes compared with the whole kidney. However, this comparison did not respect overall protein amounts, and protein amounts are significantly different between podocytes and non-podocytes (Figure 3A). Thus, a mere relative incorporation rate is not sufficient to estimate how much “energy” a podocyte spends to maintain the specific protein compared with another cell type; this would crucially depend on the copy numbers and, as an ancillary factor, on the size of the protein. Therefore, we corrected the incorporation rates by total protein abundance and size (Experimental Procedures). The analysis revealed that actin-binding proteins and cytoskeletal proteins incorporated heavy amino acids markedly stronger (Figure 5F). Interestingly, four proteins associated with hereditary podocyte disease were in the upper quartile of most dynamic proteins, among these podocin (Nphs2), Cd2ap (Cd2ap), and actinin-4 (Actn4) (Figures 5F and 5G) and Magi2 (Bierzynska et al., 2016; Figure 5F). The number of amino acids incorporated into mitochondrial proteins was not different in podocytes compared with non-podocytes (Figure 5G), which could be explained by low mitochondrial mass (Figure 3) but high turnover of these organelles (Figure 5B). These data indicate that some of the podocyte’s unique proteotypic features are partially compensated by increased protein synthesis and, potentially, proteostasis.
An unexpected finding was that podocytes have high mitochondrial protein synthesis but an overall low protein copy number of mitochondrial proteins. We asked whether this balance would tilt further when genetic manipulation of podocyte proteostasis was performed. We performed quantitative pulse labeling of amino acids in young control mice as well as in young mice with podocyte-specific knockout of autophagy-related 5 (ATG5 podocyte specific knockout [pKO]), a major positive regulator of autophagy in general and especially in podocytes (Hartleben et al., 2010; Table S6). The ATG5 pKO allele makes podocytes extremely susceptible to damage (Hartleben et al., 2010). We observed that ATG5 knockout podocytes had a significantly lower incorporation of endoplasmic reticulum proteins, a finding expected from a defect in autophagy, and, surprisingly a significantly higher incorporation of mitochondrial proteins compared with control podocytes (Figure 5H). This was consistent with 2D GO enrichment time points after different time points (Figures S6D and S6E). Isotope-labeled lysine incorporation in non-podocyte cells was not changed dramatically across both conditions (Figure 5I). In conclusion, these data delineate a specific perturbation in isotope incorporation in podocytes, specifically affecting mitochondria.
Multi-omics Integration Reveals Candidate Genes for Podocyte Function in Humans
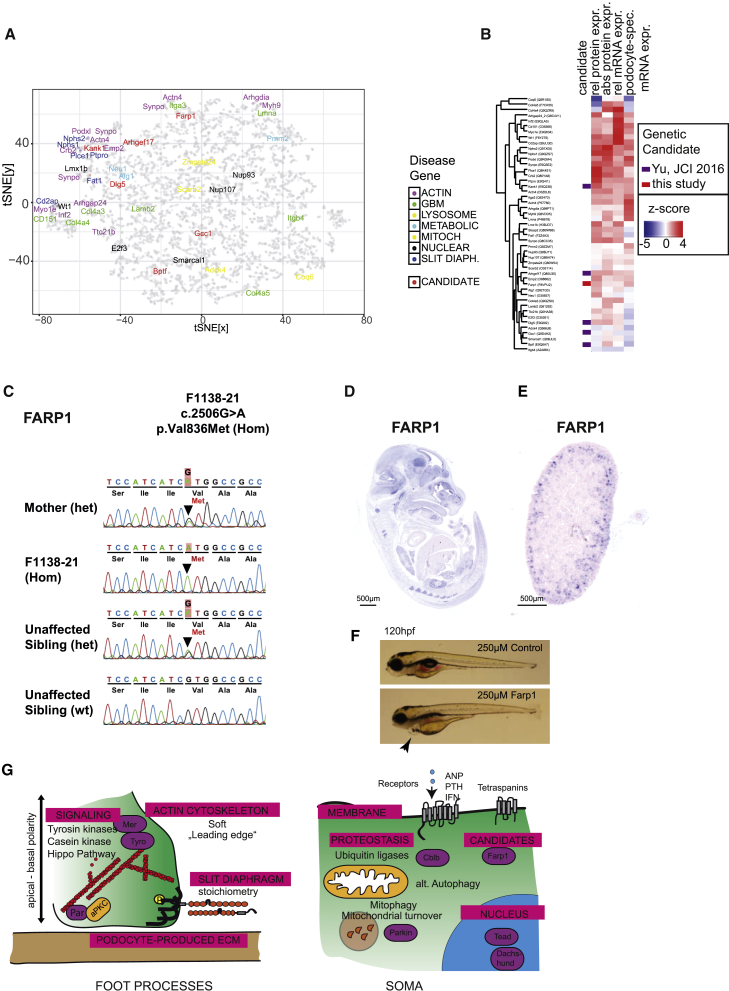
Finally, we asked whether the integration of genomic and proteomic analyses could reveal candidate genes essential for podocyte function. To this end, we performed clustering analysis of z-normalized relative mRNA expression levels, relative protein expression levels, absolute protein expression levels, and tissue specificity of podocyte mRNA levels. We found that disease-associated genes in slit diaphragm and actin-related processes were largely defined by very high Z scores in all of the four parameters and clustered close to each other (Figures 6A and 6B). Subsequently, we defined 280 genes based on each of these criteria (Table S7) and screened exome sequencing of 430 families with hereditary nephrotic syndrome for homozygous mutations in these genes. As a result, we identified a homozygous mutation in the gene FARP1 in one family with nephrotic syndrome (Table S7). This candidate gene, and the genes identified in a recent population-based study (Yu et al., 2016), clustered close to known disease genes (Figure 6A), and all shared high Z scores in at least three of the four parameters analyzed (Figure 6B). From a genetic perspective, several factors suggest potential pathogenicity of the identified allele: the mutation affects amino acid residues that are highly conserved among orthologs across evolution; in a control database of 80,000 predominantly healthy individuals (Exome Aggregation Consortium [ExAC]), the minor allele frequency of either allele is below 0.01%, and it has not been reported in the homozygous state; and the allele segregates with the affect status within the family (Figure 6C). The mutated residues were highly conserved and the mutation localized in a functional PH domain of the protein, and histology and electron microscopy showed clear signs of podocyte dysfunction within the affected individual (Figures S7A–S7E). A second patient with previously non-identified FARP1 variants was identified in a second cohort of 700 patients with nephrotic syndrome (patient was heterozygous for T789M, and c.-20T > A); however, it was unclear whether the alleles segregated with the phenotype because the DNA of family members could not be sequenced. The FARP1 gene newly identified in this study also localized predominantly to glomeruli and their precursors in development and kidneys of newborn mice (Figures 6D and 6E). In addition, knockdown of the farp1 mRNA in zebrafish larvae resulted in a phenotype of pericardial edema as well as proteinuria, indicating loss of glomerular barrier function in D. rerio (Figure 6; Figures S7F and S7G).
Figure 6.
Identification of Candidates for Human Nephrotic Syndrome Disease-Causing Genes
(A) Clustering analysis (t-SNE) of 6,700 proteins for which absolute protein expression, relative protein expression, mRNA expression, and tissue-specific mRNA expression were available.
(B) Heatmap analysis of expression of disease genes and one previously unreported candidate gene, FARP1, as well as five candidates from a previous study (Yu et al., 2016).
(C) Sanger sequencing of the respective regions of FARP1 demonstrates segregation of the mutated alleles with the affected status in family F1138 (FARP1).
(D and E) Embryonic and renal expression profile of the candidate protein Farp1 by in situ hybridization.
(D) Farp1 shows high expression in metanephric glomerular precursors and readily detectable expression in neuronal tissues and, to some extent, in other pulmonary epithelial structures in embryonic day 14.5 (E14.5) mouse embryos.
(E) Farp1 expression is maintained in murine P1 kidneys in glomeruli.
(F) Knockdown of farp1 in zebrafish induces edema and pericardial effusion (arrow), indicating proteinuria and podocyte dysfunction.
(G) Overview of insights into podocyte biology obtained from this study.
Discussion
Postmitotic specialized cell types, such as neurons or podocytes, are particularly vulnerable to age-dependent degeneration. In fact, the podocyte has emerged as a central gatekeeper to prevent proteinuria and renal failure, two unmet and frequent clinical symptoms associated with dramatically increased cardiovascular risk. Interrogation of individual cell types at multiple omics levels can yield insights into both essential molecular components and preventive strategies. Here we gained a near-comprehensive view of the static and dynamic podocyte proteome and transcriptome by applying recently developed technologies (Kulak et al., 2014). Single-cell podocyte sorting previously enabled only incomplete in vivo analysis of the podocyte proteome (Boerries et al., 2013, Rinschen et al., 2015a, Rinschen et al., 2016a). At least 10,000 proteins are suggested to be expressed in a cultured cell line (Kulak et al., 2014, Mann et al., 2013, Nagaraj et al., 2011), and a similar number of proteins has been shown to be expressed in native neurons (Sharma et al., 2015). Here we demonstrate that such a comprehensive atlas of protein and mRNA expression datasets now allows distilling molecular characteristic features of podocytes, ultimately up to the prediction of candidate genes associated with human disease. Figure 6G gives an overview of the findings in this study.
Podocytes express a high abundance of actin-binding proteins and proteins known to be localizing to the leading edge of the cell (Figures 1, 2, and 3; Table S5). Expressed in simplified terms, many of these proteins oppose the formation of stress fibers by promoting actin branching to promote a “motile” phenotype (Higgs and Pollard, 2001). The leading edge is a long-established model to study the cell biology of podocyte foot processes, and eventual slit diaphragm signaling (Garg and Holzman, 2012, Garg et al., 2010, Moeller et al., 2004), and based on these global proteomic data, this paradigm could be further expanded. This study discovered signaling molecules enriched in podocytes, which may be directly translated to novel experimental studies. Several of the proteins, but by far not all of them, have already been studied in podocytes and have been linked in part by dramatic phenotypes in conditional and targeted mouse models (e.g., protein kinase C) (Huber et al., 2009) or human patients (e.g., MAGI2; Bierzynska et al., 2016). A significant fraction of these proteins, however, has not been extensively studied and will require further consideration (Table 1; Table S4); for instance, specific members of the tetraspanin family, which were confirmed in Drosophila nephrocyte studies as highly conserved functional regulators of filtering cells (Sachs et al., 2012; Figure 3).
In addition to using these data as a resource, one may also delineate “systems-level” features of molecular podocyte identity. To demonstrate this approach in principle, we delineated differences to the proteome of a commonly used immortalized mouse podocyte cell culture line (Rinschen et al., 2016b). Cultured podocytes are widely used to investigate podocyte damage and serve as a model to study the regulation of the cytoskeleton and many other aspects of podocyte biology (Lee et al., 2015a, Shankland, 2006, Yu et al., 2013). Surprisingly, the systems-level comparison of proteome-wide expression data (Figure 4) revealed that proteins involved in stress fiber formation are among the strongest dysregulated processes in cell cultures, a finding that could be caused by stiffness of the cell culture dish (Gilbert et al., 2010). As a proof-of-principle experiment, the cultivation of either human or mouse podocyte cells on soft matrices resulted in a major shift in the proteome, which partly reduced this actin-related cell culture artefact (Figure 4). This is consistent with observations that podocytes strongly react to other mechanical cues, such as hydrostatic pressure (Kriz and Lemley, 2015), stretch (Durvasula et al., 2004, Kim et al., 2013, Petermann et al., 2005), or membrane curvature (Inoue and Ishibe, 2015), in vitro and in vivo (Hu et al., 2017, Rinschen et al., 2017). Cultivation on soft matrices does not, however, increase the expression of podocyte-specific actin binders or podocyte membrane proteins, which are only minimally expressed under both conditions.
Mitochondrial proteins are rarely expressed in podocytes (Figure 3), but the synthesis of these proteins (as measured by in vivo stable isotope pulse labeling) is faster than in non-podocytes. Therefore, the podocyte has an overall low abundance of mitochondrial proteins but high mitochondrial resynthesis and, assuming a steady-state equilibrium, also turnover. This may be a surrogate readout of predominant mitochondrial degradation (e.g., by mitophagy) in podocytes compared with other glomerular cell types. Consistently, podocytes show protein-level enrichment for the ubiquitin ligase Parkin2 (log2 fold change 2.33), a key molecule controlling mitophagy (Narendra et al., 2008; Table S2). Interestingly, when knocking out ATG5, a protein essential for autophagy (Hartleben et al., 2010), the podocyte reacts, when still intact and functional, with even further increased synthesis of mitochondrial proteins (Figure 5H). One could speculate that this finding may be caused by increased alternative autophagy and mitophagy, a process significantly increased in the absence of ATG5 (Hirota et al., 2015). In fact, mediators of alternative autophagy (e.g., Rab9 and Becn1) are abundantly expressed in podocytes but not significantly enriched (Lee et al., 2015b; Table S2). Although these findings need further corroboration, these data demonstrate the feasibility to dissolve dynamic proteostatic shifts in vivo by integrating dynamic and static protein quantifications and cutting through the various dimensions of podocyte proteostasis.
In summary, these data provide a unique resource and starting point for the next level of systematic understanding of podocyte identity, function, and glomerular disease mechanisms, including a systems view of podocyte proteome dynamics (Figure 6G). Using a multidimensional analysis, we showed that a majority of podocyte disease genes (mainly slit diaphragm- and actin-related genes) have rather high relative and absolute protein expression as well as podocyte-specific mRNA expression (Figures 6A and 6B). This opens the door for a potential integrative “reverse genetic” approach to predict functionally relevant candidate genes associated with human podocyte diseases (Figure 6; Table S7) and prioritize them for further functional testing, in particular when more advanced bioinformatics methods are utilized and further data (e.g., transcriptome data from patient cohorts) are integrated. The concept presented here will be advantageous for understanding cell-type-specific function in health and disease.
Experimental Procedures
Animals
Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J mice were purchased from The Jackson Laboratory (Bar Harbour, Massachusetts, USA) (Muzumdar et al., 2007), and Tg(NPHS2-cre)295Lbh mice were a generous gift from M. Möller (University of Aachen, Aachen, Germany). Use of Atg5flox mice has been reported previously (Hartleben et al., 2010). In general, Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J; Tg(NPHS2-cre)295Lbh mice (for deep proteome, RNA-seq, and pulsed in vivo stable isotope labeling experiments) as well as Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J; Tg(NPHS2-cre)295Lbh;Atg5flox/flox mice (pulsed in vivo stable isotope labeling experiments) were on a mixed genetic background (Sv129/C57Bl6/ICR). Breeding and genotyping was done according to standard procedures. Mice were housed in an SPF facility with free access to chow and water and a 12-hr day/night cycle. All animal experiments were conducted according to the guidelines of the American Physiological Society as well as the German law for the welfare of animals and were approved by local authorities (Regierungspräsidium Freiburg X12/06J, G11-38, and G11-34). Glomeruli and, subsequently, podocytes were isolated using essential the same method as described previously (Boerries et al., 2013). For details, see Glomerular Isolation and Podocyte Preparation in the Supplemental Experimental Procedures.
Liquid Chromatography-Tandem Mass Spectrometry Acquistion
For details regarding sample preparation of native podocytes for proteomic analysis, see the Supplemental Experimental Procedures. Analysis of peptides was performed using a quadrupole-orbitrap mass spectrometer (Q Exactive Plus, Thermo Scientific, Bremen) coupled to a nano-liquid chromatography (nLC) device. The mass spectrometers were calibrated weekly. Briefly, peptides were separated by nLC using a 4-hr gradient (flow rate, 200 nL/min). Peptides were separated on an in-house packed 50-cm column with 1.7-μm C18 beads (Dr. Maisch). Ascending concentrations of buffer B (80% acetonitrile, 0.1% formic acid) over buffer A (0.1% formic acid) were used. Then peptides were sprayed into the mass spectrometer by electron spray ionization. The acquisition parameters for the mass spectrometer were as described previously (Bartram et al., 2016). 1E6 was the AGC target for MS1. The resolution was 70,000 (mass range, 200–1200 mz−1). Tandem mass spectrometry (MS/MS) spectra of the top 10 most intense peaks were obtained by higher-energy collisional dissociation fragmentation. Resolution for the MS/MS spectra was 35,000 at 200 mz−1, AGC target to 5E5.
Cell Culture and Proteomics Analysis
Validated cell lines were regularly tested for mycoplasms using a commercial kit (VENOR, Sigma). Human podocytes (Saleem et al., 2002) were cultured in dishes as described previously at 33°C (Rinschen et al., 2016a). They were seeded on collagen-coated soft matrices (Matrigen) for 2 days (passage ∼20). Similarly, mouse podocytes (at 33°C) (Griffin et al., 2004) were cultured in coated Primaria cell culture dishes (Falcon) as described previously and seeded on collagen-coated soft matrices (Matrigen) for 2 days. For details regarding sample preparation, proteomics, and bioinformatics analysis, see the Supplemental Experimental Procedures.
Pulsed In Vivo Stable Isotope Labeling
Stable isotope labeling of animals was performed as described previously by Krüger et al. (2008). The Lys(0)-stable isotope labeling by amino acid in cell culture (SILAC)-Mouse control (12C6-lysine, “light”) and Lys(6)-SILAC-Mouse SILAC (13C6-lysine, 97%, “heavy”) mouse diets were purchased from Silantes (Martinsried, Germany). For details regarding sample preparation and proteomics of in vivo stable isotope-labeled tissue, see the Supplemental Experimental Procedures.
Statistics
For quantitative data, statistical tests (two tailed unpaired t test, Kolmogorov-Smirnov test, and two-way ANOVA test) were performed where appropriate and where indicated. In general, p < 0.05 was considered significant. For large-scale data, correction for multiple testing was performed as described in the respective bioinformatics method sections.
Study Participants, Whole-Exome Sequencing, Multi-gene Panel Testing, and Mutation Calling
We obtained blood samples and pedigrees following informed consent from individuals with steroid resistant nephrotic syndrome (SRNS) or their legal guardians. Approval for human subject research was obtained from the institutional review boards of the University of Michigan and Boston Children’s Hospital. For details regarding whole-exome sequencing, multi-gene panel testing, and mutation calling, see the Supplemental Experimental Procedures.
Model Organism Experiments
The detailed methods for functional analysis of zebrafish and Drosophila proteinuric phenotypes as well as mouse in situ hybridization experiments can be found in the Supplemental Experimental Procedures.
Acknowledgments
We thank Ruth Herzog, Charlotte Meyer, Temel Kilic, Valerie Oberüber, Christine Gretzmeier, and Barbara Joch for expert technical assistance and all members of our laboratories for helpful discussions. This study was supported by the German Research Foundation (DFG) UoC postdoctoral grant and a DFG fellowship (Ri 2811/1-1) (to M.M.R.), CRC 1140 (to F.G., C.B., and T.B.H.), KFO 329 (to T.B. and B.S.) CRC 992 (to T.B.H.), the Heisenberg program (to T.B.H.), and HU 1016/5-1 and HU 1016/8-1 (to T.B.H.); by a European Research Council (ERC) grant (to T.B.H.) and by the H2020-IMI2 Consortium BEAt-DKD (115974 to T.B.H.); by BMBF STOP-FSGS 01GM1518C (to T.B.H.); by the Excellence Initiative of the German Federal and State Governments (BIOSS) (to T.B.H.) and the Freiburg Institute for Advanced Studies (FRIAS) (to T.B.H.); and by the Else Kröner Fresenius Stiftung, NAKSYS (to T.B.H.). C.B. was supported by the Federal Ministry of Education and Research (BMBF, 01GM1515C, Project 2.3). M.B. is funded by BMBF within the framework of the e:Med Research and Funding Concept (DeCaRe, FKZ 01ZX1409B) and by DFG Collaborative Research Center (CRC) 850, Projects Z1 and C9. H.B. acknowledges funding through the DFG Excellence Cluster EXC 306. M.S. was supported by the Fritz Thyssen Foundation (10.16.2.026MN) and BMBF Grant 01GM1518A. F.H. was supported by the NIH (R01-DK076683). M.G. was supported by the German Society of Nephrology (Forschungsstipendium der Deutschen Gesellschaft für Nephrologie 2012). We thank the Yale Center for Mendelian Genomics (U54HG006504) for whole-exome sequencing.
Author Contributions
M.M.R., M.G., F.G., T.B., and T.B.H. designed the research studies. M.M.R., M.G., F.G., S.Z., M.H., O.K., M.Z., D.A.B., S.D., C.P., C.T., H.Y.G., and V.K. conducted experiments. G.D., M.P., D.A.B., E. K., C. B., and F.H. acquired genetic data. M.S. and P.S. performed zebrafish experiments. M.M.R., M.G., B.S., M.B., H.B., J.D., and T.B.H. analyzed data. M.K. and J.D. provided reagents and novel tools. M.M.R., M.G., F.G., T.B., and T.B.H. wrote the manuscript.
Declaration of Interests
F.H. is a founder of Goldfinch-Bio and a member of its scientific advisory board. C.B. and E.K. are employees of Bioscientia/Sonic Healthcare. C.B. holds a part-time faculty appointment at the University of Freiburg.
Published: May 22, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and seven tables and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.04.059.
Contributor Information
Markus M. Rinschen, Email: markus.rinschen@uk-koeln.de.
Thomas Benzing, Email: thomas.benzing@uk-koeln.de.
Tobias B. Huber, Email: t.huber@uke.de.
Supplemental Information
The file lists all proteins discovered in native podocytes using the SEQUEST algorithm.
The file lists relative (LFQ) and absolute (iBAQ) quantification values for podocytes and non-podocytes.
The file lists expression values for transcripts within native podocytes and non-podocytes. Used raw files are in the second tab of the excel sheet.
The different tabs list kinases, receptors, proteases, phosphatases, ubiquitin ligases, actin binders, and transcription factors shown to be enriched in native podocytes.
The file lists averaged Heavy/Light (H/L) ratios for isotope labeled lysine incorporation. The second tab lists results for proteomic analysis ATG5 podocyte-specific knockout mice and control mice.
280 candidate protein-gene pairs for genetic analysis and information on the FARP1 patient. The first tab of the table depicts gene symbols of the selected candidates. The second tab of the table gives detailed information on the FARP1 index patient.
References
- Bartram M.P., Habbig S., Pahmeyer C., Höhne M., Weber L.T., Thiele H., Altmüller J., Kottoor N., Wenzel A., Krueger M. Three-layered proteomic characterization of a novel ACTN4 mutation unravels its pathogenic potential in FSGS. Hum. Mol. Genet. 2016;25:1152–1164. doi: 10.1093/hmg/ddv638. [DOI] [PubMed] [Google Scholar]
- Bierzynska A., Soderquest K., Koziell A. Genes and podocytes - new insights into mechanisms of podocytopathy. Front. Endocrinol. (Lausanne) 2015;5:226. doi: 10.3389/fendo.2014.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierzynska A., Soderquest K., Dean P., Colby E., Rollason R., Jones C., Inward C.D., McCarthy H.J., Simpson M.A., Lord G.M. MAGI2 Mutations Cause Congenital Nephrotic Syndrome. J. Am. Soc. Nephrol. 2016;28:1614–1621. doi: 10.1681/ASN.2016040387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerries M., Grahammer F., Eiselein S., Buck M., Meyer C., Goedel M., Bechtel W., Zschiedrich S., Pfeifer D., Laloë D. Molecular fingerprinting of the podocyte reveals novel gene and protein regulatory networks. Kidney Int. 2013;83:1052–1064. doi: 10.1038/ki.2012.487. [DOI] [PubMed] [Google Scholar]
- Boute N., Gribouval O., Roselli S., Benessy F., Lee H., Fuchshuber A., Dahan K., Gubler M.C., Niaudet P., Antignac C. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat. Genet. 2000;24:349–354. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- Brunskill E.W., Georgas K., Rumballe B., Little M.H., Potter S.S. Defining the molecular character of the developing and adult kidney podocyte. PLoS ONE. 2011;6:e24640. doi: 10.1371/journal.pone.0024640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami E., Demir E., Schultz N., Taylor B.S., Sander C. Automated network analysis identifies core pathways in glioblastoma. PLoS ONE. 2010;5:e8918. doi: 10.1371/journal.pone.0008918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Chronic Kidney Disease (CKD) Surveillance Project. https://nccd.cdc.gov/ckd.
- Cox J., Mann M. 1D and 2D annotation enrichment: a statistical method integrating quantitative proteomics with complementary high-throughput data. BMC Bioinformatics. 2012;13(Suppl 16):S12. doi: 10.1186/1471-2105-13-S16-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durvasula R.V., Petermann A.T., Hiromura K., Blonski M., Pippin J., Mundel P., Pichler R., Griffin S., Couser W.G., Shankland S.J. Activation of a local tissue angiotensin system in podocytes by mechanical strain. Kidney Int. 2004;65:30–39. doi: 10.1111/j.1523-1755.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- Endlich N., Endlich K. cAMP pathway in podocytes. Microsc. Res. Tech. 2002;57:228–231. doi: 10.1002/jemt.10079. [DOI] [PubMed] [Google Scholar]
- Fleuren E.D.G., Hillebrandt-Roeffen M.H.S., Flucke U.E., Te Loo D.M.W.M., Boerman O.C., van der Graaf W.T.A., Versleijen-Jonkers Y.M.H. The role of AXL and the in vitro activity of the receptor tyrosine kinase inhibitor BGB324 in Ewing sarcoma. Oncotarget. 2014;5:12753–12768. doi: 10.18632/oncotarget.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J., Wei C., Lee K., Zhang W., He W., Chuang P., Liu Z., He J.C. Comparison of Glomerular and Podocyte mRNA Profiles in Streptozotocin-Induced Diabetes. J. Am. Soc. Nephrol. 2016;27:1006–1014. doi: 10.1681/ASN.2015040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg P., Holzman L.B. Podocytes: gaining a foothold. Exp. Cell Res. 2012;318:955–963. doi: 10.1016/j.yexcr.2012.02.030. [DOI] [PubMed] [Google Scholar]
- Garg P., Verma R., Cook L., Soofi A., Venkatareddy M., George B., Mizuno K., Gurniak C., Witke W., Holzman L.B. Actin-depolymerizing factor cofilin-1 is necessary in maintaining mature podocyte architecture. J. Biol. Chem. 2010;285:22676–22688. doi: 10.1074/jbc.M110.122929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P.M., Havenstrite K.L., Magnusson K.E.G., Sacco A., Leonardi N.A., Kraft P., Nguyen N.K., Thrun S., Lutolf M.P., Blau H.M. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahammer F., Schell C., Huber T.B. The podocyte slit diaphragm--from a thin grey line to a complex signalling hub. Nat. Rev. Nephrol. 2013;9:587–598. doi: 10.1038/nrneph.2013.169. [DOI] [PubMed] [Google Scholar]
- Griffin S.V., Hiromura K., Pippin J., Petermann A.T., Blonski M.J., Krofft R., Takahashi S., Kulkarni A.B., Shankland S.J. Cyclin-dependent kinase 5 is a regulator of podocyte differentiation, proliferation, and morphology. Am. J. Pathol. 2004;165:1175–1185. doi: 10.1016/S0002-9440(10)63378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurkan S., Cabinian A., Lopez V., Bhaumik M., Chang J.-M., Rabson A.B., Mundel P. Inhibition of type I interferon signalling prevents TLR ligand-mediated proteinuria. J. Pathol. 2013;231:248–256. doi: 10.1002/path.4235. [DOI] [PubMed] [Google Scholar]
- Harita Y., Kurihara H., Kosako H., Tezuka T., Sekine T., Igarashi T., Hattori S. Neph1, a component of the kidney slit diaphragm, is tyrosine-phosphorylated by the Src family tyrosine kinase and modulates intracellular signaling by binding to Grb2. J. Biol. Chem. 2008;283:9177–9186. doi: 10.1074/jbc.M707247200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartleben B., Gödel M., Meyer-Schwesinger C., Liu S., Ulrich T., Köbler S., Wiech T., Grahammer F., Arnold S.J., Lindenmeyer M.T. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J. Clin. Invest. 2010;120:1084–1096. doi: 10.1172/JCI39492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs H.N., Pollard T.D. Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annu. Rev. Biochem. 2001;70:649–676. doi: 10.1146/annurev.biochem.70.1.649. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Yamashita S., Kurihara Y., Jin X., Aihara M., Saigusa T., Kang D., Kanki T. Mitophagy is primarily due to alternative autophagy and requires the MAPK1 and MAPK14 signaling pathways. Autophagy. 2015;11:332–343. doi: 10.1080/15548627.2015.1023047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgin J.B., Nair V., Zhang H., Randolph A., Harris R.C., Nelson R.G., Weil E.J., Cavalcoli J.D., Patel J.M., Brosius F.C., 3rd, Kretzler M. Identification of cross-species shared transcriptional networks of diabetic nephropathy in human and mouse glomeruli. Diabetes. 2013;62:299–308. doi: 10.2337/db11-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M., Azeloglu E.U., Ron A., Tran-Ba K.-H., Calizo R.C., Tavassoly I., Bhattacharya S., Jayaraman G., Chen Y., Rabinovich V. A biomimetic gelatin-based platform elicits a pro-differentiation effect on podocytes through mechanotransduction. Sci. Rep. 2017;7:43934. doi: 10.1038/srep43934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber T.B., Hartleben B., Winkelmann K., Schneider L., Becker J.U., Leitges M., Walz G., Haller H., Schiffer M. Loss of podocyte aPKClambda/iota causes polarity defects and nephrotic syndrome. J. Am. Soc. Nephrol. 2009;20:798–806. doi: 10.1681/ASN.2008080871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Ishibe S. Podocyte endocytosis in the regulation of the glomerular filtration barrier. Am. J. Physiol. Renal Physiol. 2015;309:F398–F405. doi: 10.1152/ajprenal.00136.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey P.A., Miller R.T. Mechanisms of mechanical signaling in development and disease. J. Cell Sci. 2011;124:9–18. doi: 10.1242/jcs.071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju W., Smith S., Kretzler M. Genomic biomarkers for chronic kidney disease. Transl. Res. 2012;159:290–302. doi: 10.1016/j.trsl.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kann M., Ettou S., Jung Y.L., Lenz M.O., Taglienti M.E., Park P.J., Schermer B., Benzing T., Kreidberg J.A. Genome-Wide Analysis of Wilms’ Tumor 1-Controlled Gene Expression in Podocytes Reveals Key Regulatory Mechanisms. J. Am. Soc. Nephrol. 2015;26:2097–2104. doi: 10.1681/ASN.2014090940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J.M., Kim S.H., North K.N., Rennke H., Correia L.A., Tong H.Q., Mathis B.J., Rodríguez-Pérez J.C., Allen P.G., Beggs A.H., Pollak M.R. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat. Genet. 2000;24:251–256. doi: 10.1038/73456. [DOI] [PubMed] [Google Scholar]
- Kestilä M., Lenkkeri U., Männikkö M., Lamerdin J., McCready P., Putaala H., Ruotsalainen V., Morita T., Nissinen M., Herva R. Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol. Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- Kim E.Y., Anderson M., Wilson C., Hagmann H., Benzing T., Dryer S.E. NOX2 interacts with podocyte TRPC6 channels and contributes to their activation by diacylglycerol: essential role of podocin in formation of this complex. Am. J. Physiol. Cell Physiol. 2013;305:C960–C971. doi: 10.1152/ajpcell.00191.2013. [DOI] [PubMed] [Google Scholar]
- Kim M.-S., Pinto S.M., Getnet D., Nirujogi R.S., Manda S.S., Chaerkady R., Madugundu A.K., Kelkar D.S., Isserlin R., Jain S. A draft map of the human proteome. Nature. 2014;509:575–581. doi: 10.1038/nature13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli P., Bartram M.P., Habbig S., Pahmeyer C., Lamkemeyer T., Benzing T., Schermer B., Rinschen M.M. Label-free quantitative proteomic analysis of the YAP/TAZ interactome. Am. J. Physiol. Cell Physiol. 2014;306:C805–C818. doi: 10.1152/ajpcell.00339.2013. [DOI] [PubMed] [Google Scholar]
- Köttgen A., Pattaro C., Böger C.A., Fuchsberger C., Olden M., Glazer N.L., Parsa A., Gao X., Yang Q., Smith A.V. New loci associated with kidney function and chronic kidney disease. Nat. Genet. 2010;42:376–384. doi: 10.1038/ng.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriz W., Lemley K.V. A potential role for mechanical forces in the detachment of podocytes and the progression of CKD. J. Am. Soc. Nephrol. 2015;26:258–269. doi: 10.1681/ASN.2014030278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger M., Moser M., Ussar S., Thievessen I., Luber C.A., Forner F., Schmidt S., Zanivan S., Fässler R., Mann M. SILAC mouse for quantitative proteomics uncovers kindlin-3 as an essential factor for red blood cell function. Cell. 2008;134:353–364. doi: 10.1016/j.cell.2008.05.033. [DOI] [PubMed] [Google Scholar]
- Kulak N.A., Pichler G., Paron I., Nagaraj N., Mann M. Minimal, encapsulated proteomic-sample processing applied to copy-number estimation in eukaryotic cells. Nat. Methods. 2014;11:319–324. doi: 10.1038/nmeth.2834. [DOI] [PubMed] [Google Scholar]
- Lee H.W., Khan S.Q., Faridi M.H., Wei C., Tardi N.J., Altintas M.M., Elshabrawy H.A., Mangos S., Quick K.L., Sever S. A Podocyte-Based Automated Screening Assay Identifies Protective Small Molecules. J. Am. Soc. Nephrol. 2015;26:2741–2752. doi: 10.1681/ASN.2014090859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.W., Chou C.-L., Knepper M.A. Deep Sequencing in Microdissected Renal Tubules Identifies Nephron Segment-Specific Transcriptomes. J. Am. Soc. Nephrol. 2015;26:2669–2677. doi: 10.1681/ASN.2014111067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Sherick A.B., Zhang W., Menachof K.K., Hill A.A., Rinella S., Kirkpatrick G., Page L.S., Stashko M.A., Jordan C.T., Wei Q. Efficacy of a Mer and Flt3 tyrosine kinase small molecule inhibitor, UNC1666, in acute myeloid leukemia. Oncotarget. 2015;6:6722–6736. doi: 10.18632/oncotarget.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Beyer A., Aebersold R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell. 2016;165:535–550. doi: 10.1016/j.cell.2016.03.014. [DOI] [PubMed] [Google Scholar]
- Mann M., Kulak N.A., Nagaraj N., Cox J. The coming age of complete, accurate, and ubiquitous proteomes. Mol. Cell. 2013;49:583–590. doi: 10.1016/j.molcel.2013.01.029. [DOI] [PubMed] [Google Scholar]
- Meguid El Nahas A., Bello A.K. Chronic kidney disease: the global challenge. Lancet. 2005;365:331–340. doi: 10.1016/S0140-6736(05)17789-7. [DOI] [PubMed] [Google Scholar]
- Moeller M.J., Soofi A., Braun G.S., Li X., Watzl C., Kriz W., Holzman L.B. Protocadherin FAT1 binds Ena/VASP proteins and is necessary for actin dynamics and cell polarization. EMBO J. 2004;23:3769–3779. doi: 10.1038/sj.emboj.7600380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar M.D., Tasic B., Miyamichi K., Li L., Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Nagaraj N., Wisniewski J.R., Geiger T., Cox J., Kircher M., Kelso J., Pääbo S., Mann M. Deep proteome and transcriptome mapping of a human cancer cell line. Mol. Syst. Biol. 2011;7:548. doi: 10.1038/msb.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D., Tanaka A., Suen D.-F., Youle R.J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New L.A., Martin C.E., Jones N. Advances in slit diaphragm signaling. Curr. Opin. Nephrol. Hypertens. 2014;23:420–430. doi: 10.1097/01.mnh.0000447018.28852.b6. [DOI] [PubMed] [Google Scholar]
- Pavenstädt H., Kriz W., Kretzler M. Cell biology of the glomerular podocyte. Physiol. Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- Petermann A.T., Pippin J., Durvasula R., Pichler R., Hiromura K., Monkawa T., Couser W.G., Shankland S.J. Mechanical stretch induces podocyte hypertrophy in vitro. Kidney Int. 2005;67:157–166. doi: 10.1111/j.1523-1755.2005.00066.x. [DOI] [PubMed] [Google Scholar]
- Reiser J., Polu K.R., Möller C.C., Kenlan P., Altintas M.M., Wei C., Faul C., Herbert S., Villegas I., Avila-Casado C. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat. Genet. 2005;37:739–744. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinschen M.M., Wu X., König T., Pisitkun T., Hagmann H., Pahmeyer C., Lamkemeyer T., Kohli P., Schnell N., Schermer B. Phosphoproteomic Analysis Reveals Regulatory Mechanisms at the Kidney Filtration Barrier. J. Am. Soc. Nephrol. 2014;25:1509–1522. doi: 10.1681/ASN.2013070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinschen M.M., Benzing T., Limbutara K., Pisitkun T. Proteomic analysis of the kidney filtration barrier--Problems and perspectives. Proteomics Clin. Appl. 2015;9:1053–1068. doi: 10.1002/prca.201400201. [DOI] [PubMed] [Google Scholar]
- Rinschen M.M., Pahmeyer C., Pisitkun T., Schnell N., Wu X., Maaß M., Bartram M.P., Lamkemeyer T., Schermer B., Benzing T. Comparative phosphoproteomic analysis of mammalian glomeruli reveals conserved podocin C-terminal phosphorylation as a determinant of slit diaphragm complex architecture. Proteomics. 2015;15:1326–1331. doi: 10.1002/pmic.201400235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinschen M.M., Bharill P., Wu X., Kohli P., Reinert M.J., Kretz O., Saez I., Schermer B., Höhne M., Bartram M.P. The ubiquitin ligase Ubr4 controls stability of podocin/MEC-2 supercomplexes. Hum. Mol. Genet. 2016;25:1328–1344. doi: 10.1093/hmg/ddw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinschen M.M., Schroeter C.B., Koehler S., Ising C., Schermer B., Kann M., Benzing T., Brinkkoetter P.T. Quantitative deep-mapping of the cultured podocyte proteome uncovers shifts in proteostatic mechanisms during differentiation. Am. J. Physiol. Cell Physiol. 2016;311:C404–C417. doi: 10.1152/ajpcell.00121.2016. [DOI] [PubMed] [Google Scholar]
- Rinschen M.M., Grahammer F., Hoppe A.-K., Kohli P., Hagmann H., Kretz O., Bertsch S., Höhne M., Göbel H., Bartram M.P. YAP-mediated mechanotransduction determines the podocyte’s response to damage. Sci. Signal. 2017;10 doi: 10.1126/scisignal.aaf8165. eaaf8165. [DOI] [PubMed] [Google Scholar]
- Sachs N., Claessen N., Aten J., Kreft M., Teske G.J.D., Koeman A., Zuurbier C.J., Janssen H., Sonnenberg A. Blood pressure influences end-stage renal disease of Cd151 knockout mice. J. Clin. Invest. 2012;122:348–358. doi: 10.1172/JCI58878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski C.E., Lovric S., Ashraf S., Pabst W.L., Gee H.Y., Kohl S., Engelmann S., Vega-Warner V., Fang H., Halbritter J., SRNS Study Group A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J. Am. Soc. Nephrol. 2015;26:1279–1289. doi: 10.1681/ASN.2014050489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem M.A., O’Hare M.J., Reiser J., Coward R.J., Inward C.D., Farren T., Xing C.Y., Ni L., Mathieson P.W., Mundel P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- Sampson M.G., Hodgin J.B., Kretzler M. Defining nephrotic syndrome from an integrative genomics perspective. Pediatr. Nephrol. Berl. Ger. 2015;30:51–63. doi: 10.1007/s00467-014-2857-9. quiz 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savas J.N., Toyama B.H., Xu T., Yates J.R., 3rd, Hetzer M.W. Extremely long-lived nuclear pore proteins in the rat brain. Science. 2012;335:942. doi: 10.1126/science.1217421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieder S., Nagai M., Orlando R.A., Takeda T., Farquhar M.G. Podocalyxin activates RhoA and induces actin reorganization through NHERF1 and Ezrin in MDCK cells. J. Am. Soc. Nephrol. 2004;15:2289–2298. doi: 10.1097/01.ASN.0000135968.49899.E8. [DOI] [PubMed] [Google Scholar]
- Schwanhäusser B., Busse D., Li N., Dittmar G., Schuchhardt J., Wolf J., Chen W., Selbach M. Global quantification of mammalian gene expression control. Nature. 2011;473:337–342. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- Schwartzman M., Reginensi A., Wong J.S., Basgen J.M., Meliambro K., Nicholas S.B., D’Agati V., McNeill H., Campbell K.N. Podocyte-Specific Deletion of Yes-Associated Protein Causes FSGS and Progressive Renal Failure. J. Am. Soc. Nephrol. 2015;27:216–226. doi: 10.1681/ASN.2014090916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalom O., Shalva N., Altschuler Y., Motro B. The mammalian Nek1 kinase is involved in primary cilium formation. FEBS Lett. 2008;582:1465–1470. doi: 10.1016/j.febslet.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Shankland S.J. The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- Sharma K., Schmitt S., Bergner C.G., Tyanova S., Kannaiyan N., Manrique-Hoyos N., Kongi K., Cantuti L., Hanisch U.-K., Philips M.-A. Cell type- and brain region-resolved mouse brain proteome. Nat. Neurosci. 2015;18:1819–1831. doi: 10.1038/nn.4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffel J., Valletta D., Federlein A., Ehm K., Volkmann R., Füchsl A.M., Witzgall R., Kuhn M., Schweda F. Natriuretic Peptide Receptor Guanylyl Cyclase-A in Podocytes is Renoprotective but Dispensable for Physiologic Renal Function. J. Am. Soc. Nephrol. 2016;28:260–277. doi: 10.1681/ASN.2015070731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susztak K. Understanding the epigenetic syntax for the genetic alphabet in the kidney. J. Am. Soc. Nephrol. 2014;25:10–17. doi: 10.1681/ASN.2013050461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J., Taylor P., Moran M.F. Proteomic analysis of the epidermal growth factor receptor (EGFR) interactome and post-translational modifications associated with receptor endocytosis in response to EGF and stress. Mol. Cell. Proteomics MCP. 2014;13:1644–1658. doi: 10.1074/mcp.M114.038596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama B.H., Savas J.N., Park S.K., Harris M.S., Ingolia N.T., Yates J.R., 3rd, Hetzer M.W. Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell. 2013;154:971–982. doi: 10.1016/j.cell.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlén M., Fagerberg L., Hallström B.M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson Å., Kampf C., Sjöstedt E., Asplund A. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- Vogel C., Marcotte E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012;13:227–232. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weavers H., Prieto-Sánchez S., Grawe F., Garcia-López A., Artero R., Wilsch-Bräuninger M., Ruiz-Gómez M., Skaer H., Denholm B. The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature. 2009;457:322–326. doi: 10.1038/nature07526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm M., Schlegl J., Hahne H., Gholami A.M., Lieberenz M., Savitski M.M., Ziegler E., Butzmann L., Gessulat S., Marx H. Mass-spectrometry-based draft of the human proteome. Nature. 2014;509:582–587. doi: 10.1038/nature13319. [DOI] [PubMed] [Google Scholar]
- Wirnsberger G., Zwolanek F., Asaoka T., Kozieradzki I., Tortola L., Wimmer R.A., Kavirayani A., Fresser F., Baier G., Langdon W.Y. Inhibition of CBLB protects from lethal Candida albicans sepsis. Nat. Med. 2016;22:915–923. doi: 10.1038/nm.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiśniewski J.R., Hein M.Y., Cox J., Mann M. A “proteomic ruler” for protein copy number and concentration estimation without spike-in standards. Mol. Cell. Proteomics. 2014;13:3497–3506. doi: 10.1074/mcp.M113.037309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C.-C., Fornoni A., Weins A., Hakroush S., Maiguel D., Sageshima J., Chen L., Ciancio G., Faridi M.H., Behr D. Abatacept in B7-1-positive proteinuric kidney disease. N. Engl. J. Med. 2013;369:2416–2423. doi: 10.1056/NEJMoa1304572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Artomov M., Brähler S., Stander M.C., Shamsan G., Sampson M.G., White J.M., Kretzler M., Miner J.H., Jain S. A role for genetic susceptibility in sporadic focal segmental glomerulosclerosis. J. Clin. Invest. 2016;126:1067–1078. doi: 10.1172/JCI82592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Zhao Y., Han Z. An in vivo functional analysis system for renal gene discovery in Drosophila pericardial nephrocytes. J. Am. Soc. Nephrol. 2013;24:191–197. doi: 10.1681/ASN.2012080769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., DeRyckere D., Hunter D., Liu J., Stashko M.A., Minson K.A., Cummings C.T., Lee M., Glaros T.G., Newton D.L. UNC2025, a potent and orally bioavailable MER/FLT3 dual inhibitor. J. Med. Chem. 2014;57:7031–7041. doi: 10.1021/jm500749d. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The file lists all proteins discovered in native podocytes using the SEQUEST algorithm.
The file lists relative (LFQ) and absolute (iBAQ) quantification values for podocytes and non-podocytes.
The file lists expression values for transcripts within native podocytes and non-podocytes. Used raw files are in the second tab of the excel sheet.
The different tabs list kinases, receptors, proteases, phosphatases, ubiquitin ligases, actin binders, and transcription factors shown to be enriched in native podocytes.
The file lists averaged Heavy/Light (H/L) ratios for isotope labeled lysine incorporation. The second tab lists results for proteomic analysis ATG5 podocyte-specific knockout mice and control mice.
280 candidate protein-gene pairs for genetic analysis and information on the FARP1 patient. The first tab of the table depicts gene symbols of the selected candidates. The second tab of the table gives detailed information on the FARP1 index patient.