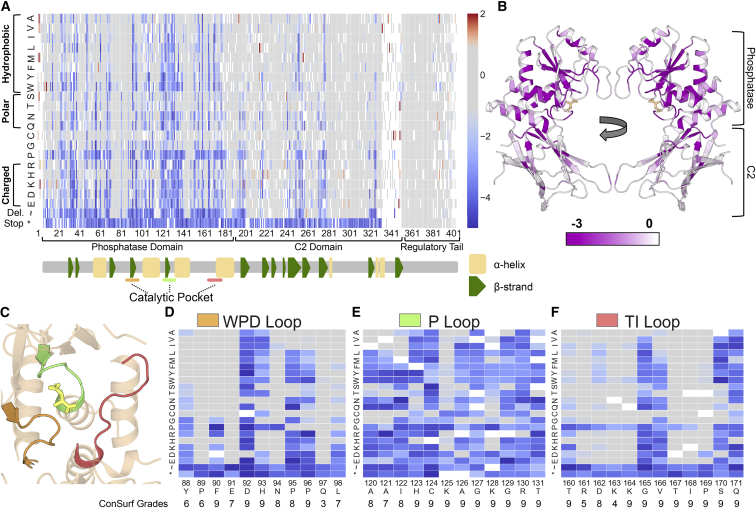
Figure 2.
High-Resolution Map of the Functional Effects of PTEN Mutations
(A) Heatmap schematic showing high-confidence fitness scores for 7,244 PTEN missense, nonsense, or in-frame deletion mutations (86% of possible). Columns are each protein position and amino acids are listed in rows ordered according to biophysical characteristics. Variants with fitness scores within the 95th percentile (two-sided) of synonymous wild-type like mutations are colored gray. Variants with fitness scores lower than the synonymous distribution are colored blue while variants with higher fitness scores are colored red. The major protein domains, as well as the secondary structure features, are indicated in the track below the heatmap (α-helices as yellow rectangles and β-strands as green pentagons).
(B) Ribbon diagram of PTEN crystal structure with residues colored by average fitness score. Darker purple corresponds to more damaging scores.
(C) Ribbon diagram highlighting the crystal structure of the PTEN catalytic pocket, composed of the WPD (orange), P (green), and TI-loops (salmon).
(D–F) The fitness scores of mutations at the residues composing the three catalytic pocket loops. Beneath each position is the Consurf grade (Material and Methods), which represents the relative evolutionary conservation, with nine being the most conserved and one being the least conserved.