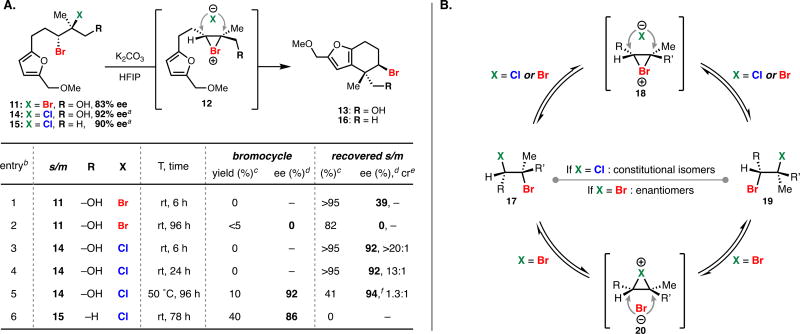
Table 1.
Timepoint analysis for the solvolytic cyclization of enantioenriched dihalides.
(A) aPrepared as a >20:1 mixture of constitutional bromochloride isomers.
1.5 equiv. K2CO3, 0.05 M in HFIP.
Based on 1H NMR analysis using 1,4-dinitrobenzene as an internal standard.
According to chiral HPLC analysis.
Ratio of constitutional isomers according to 1H NMR analysis.
HPLC integration was complicated by the presence of the corresponding constitutional isomer.
(B) Proposed mechanism for equilibration of bromochloride constitutional isomers and racemization of dibromides.