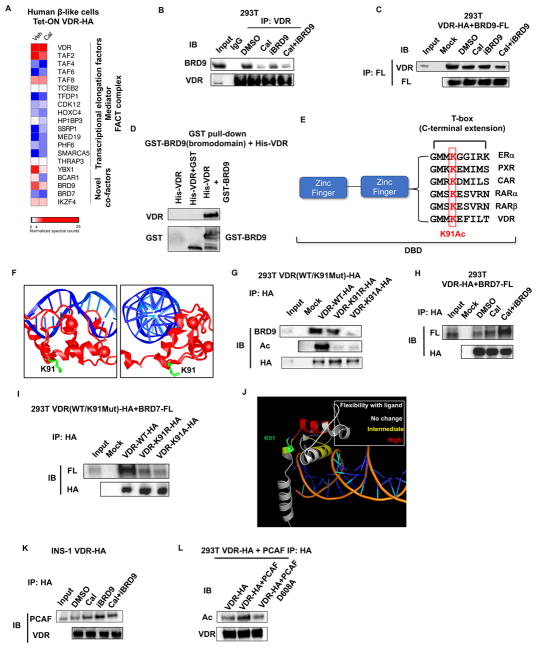
Figure 2. Bromodomain proteins BRD7 and BRD9 bind acetylated VDR.
(A) Heat map of VDR interacting proteins in human β-like cells in the absence or presence of ligand Cal. Spectral counts normalized to VDR.
(B) Western blot of BRD9 in immunoprecipitates (IP) of endogenous VDR from 293T cells 1h after indicated treatments.
(C) Western blot of ectopically expressed VDR in IP of 293T cells expressing Flag-tagged BRD9, 1h after indicated treatments.
(D) In vitro interaction of purified full-length HA-VDR with the bromodomain of BRD9 fused to GST.
(E) Alignment of T-box sequences from related nuclear receptors showing conservation of the lysine acetylated in VDR.
(F) Crystal structure of the DNA binding domains of VDR (red) bound to DNA (blue), highlighting the sidechain of K91 (green). Image is derived from the VDR crystal structure PDB ID 1kb2 (Shaffer and Gewirth, 2002).
(G) Western blot of endogenous BRD9 in IP from 293T cells expressing wild type, K91R, or K91A HA-tagged VDR.
(H) Western blot of ectopically expressed VDR in IP from 293T cells expressing Flag-tagged BRD7, 1h after indicated treatments.
(I) Western blot of ectopically expressed Flag-BRD7 in IP from 293T cells expressing wild type, K91R, or K91A HA-tagged VDR.
(J) Reported increases in hydrogen-deuterium exchange upon addition of 1,25 (OH)2 vitamin D (Zhang et al., 2011), mapped onto the crystal structure of the DNA binding domain of VDR.
(K) Western blot of endogenous PCAF in IP from INS1 cells expressing HA-VDR, 1h after indicated treatments.
(L) Western blot of ectopically expressed HA-VDR in IP from 293T cells over-expressing wild type, or an enzymatically dead PCAF (D608A).
See also Figure S3.