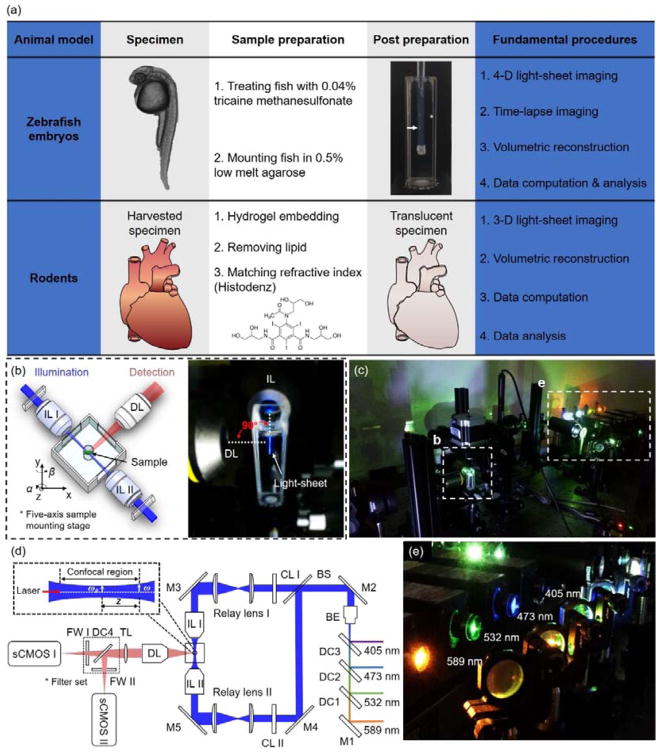
Figure 1.
Fundamental concept of the light-sheet imaging strategy. (a) The critical procedures of the multi-scale imaging are indicated for both embryonic zebrafish and mouse studies. (b) The specimen is mounted at the intersection of the illumination lens (IL) with the detection lens (DL). The laser light-sheet is excited from the IL in a 2-D plane which is orthogonal to the detection axis. The LSFM system provides a long working distance with air objective lenses in comparison to water-dipping lenses in conventional light-sheet systems. (c–d) A photo and a schematic illustrate the layout of the light-sheeting imaging system. A cylindrical lens (CL) converts the laser beam to a sheet of laser light that can transversely illuminate a thin layer of the sample. The illuminated 2-D thin layer (fluorescent detection in red) is captured by the high-frame rate sCMOS camera. The filter wheels (FW I and II) in front of sCMOS cameras are used for multi-color acquisitions. (e) A photo depicts an array of laser beams aligned for multi-channel fluorescent detection. M: mirror; BS: beam splitter; BE: beam expander; TL: tube lens; DC: dichroic mirror; FW: filter wheel.