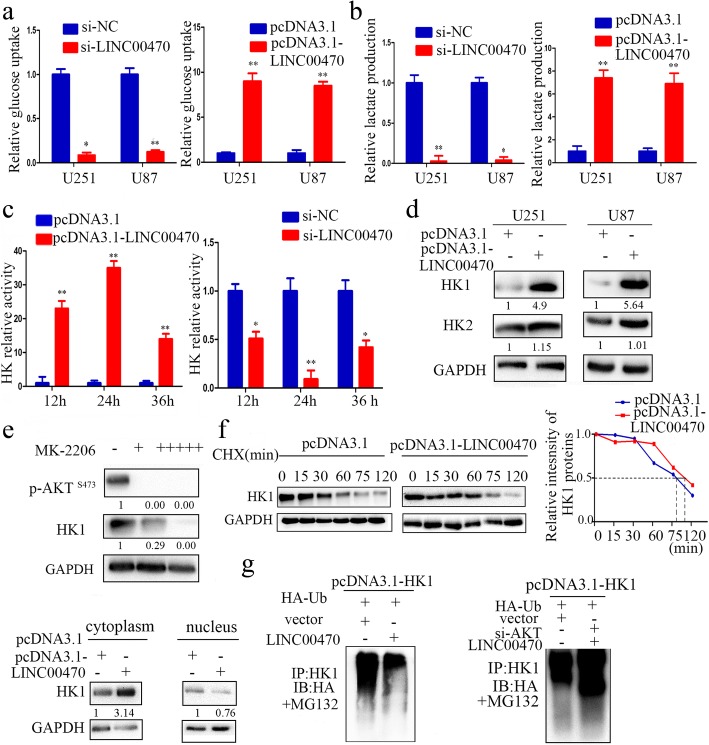
Fig. 5.
LINC00470 inhibited HK1 ubiquitination to affect glycolysis by positively regulating AKT activation. a RT-qPCR measured the expression of LINC00470 in the GBM cell lines; GBM cells were transfected with si-LINC00470 or pcDNA3.1-LINC00470. Data are presented as the mean ± S.E.M. of three independent experiments; *p < 0.05, **p < 0.01. b Relative levels of glucose uptake and lactate production were detected in GBM cells. GBM cells were transfected with si-LINC00470 or pcDNA3.1-LINC00470. Data are presented as the mean ± S.E.M. of three independent experiments; *p < 0.05, **p < 0.01. c HK activity was measured at different time points after GBM cells were transfected with pcDNA3.1-LINC00470 or si-LINC00470. Data are presented as the mean ± S.E.M. of three independent experiments; **p < 0.01, ***p < 0.001. d Western blotting detected the expression levels of HK1 and HK2 in GBM cells transfected by LINC00470. e Upper, Western blotting detected the expression levels of p-AKTS473 and HK1 in U251 cells. The cells were treated with different concentrations of AKT inhibitor (MK-2206; +, 1 μM; +++++, 5 μM); lower, Western blotting detected the expression levels of HK1 in the cytoplasm and nucleus in U251 cells transfected by pcDNA3.1-LINC00470. f The half-life of HK1 was assessed in U251 cells. Cells were transfected with pcDNA3.1-LINC00470. g The relative amount of ubiquitination HK1 was determined by a ubiquitination assay in U251 cells transfected by LINC00470 or si-AKT and LINC00470