Abstract
Objective:
Diabetic kidney disease (DKD) has become one of the major causes of end-stage renal disease. Urinary extracellular vesicles (uEVs) contain rich biological information which could be the ideal source for noninvasive biomarkers of DKD. This review discussed the potential early diagnostic and therapeutic values of proteins and microRNAs in uEVs in DKD.
Data Sources:
This review was based articles published in PubMed, Embase, Cochrane, and Google Scholar databases up to November 20, 2017, with the following keywords: “Diabetic kidney disease”, “Extracellular vesicle”, and “Urine”.
Study Selection:
Relevant articles were carefully reviewed, with no exclusions applied to the study design and publication type.
Results:
There is no “gold standard” technology to separate and/or purify uEVs. The uEVs contain a variety of proteins and RNAs and participate in the physiological and pathological processes of the kidney. UEVs, especially urinary exosomes, may be useful biomarkers for early diagnosis and treatment to DKD. Furthermore, the uEVs has been used as a therapeutic target for DKD.
Conclusion:
Proteins and nucleic acids in uEVs represent promising biomarker for the diagnosis and treatment of DKD.
Keywords: Biomarker, Diabetic Kidney Disease, Diagnosis, Extracellular Vesicles, Therapy
摘要
目的:
糖尿病肾病已经成为导致终末期肾病的一种最主要原因。尿液细胞外囊泡包含了丰富的生物信息,可以作为糖尿病肾 病非侵入性生物标记物的理想来源。本综述会讨论尿液细胞外囊泡中的蛋白质及微小RNA在早期诊断及治疗糖尿病肾病中的 潜在价值。
数据源:
本综述基于2017年11月20日前在线文献数据库的全面检索,包含PubMed, Embase, Cochrane and Google学术搜索, 检 索关键词为:“糖尿病肾病”,“细胞外囊泡”和“尿液”。
文献筛选:
相关文章已进行仔细筛选,没有因研究设计和出版类型而排除文献。
结果:
没有分离和/或纯化尿液细胞外囊泡的“金标准”技术。尿液细胞外囊泡包含大量的蛋白质和RNA,它们参与了肾脏的生 理及病理过程。尿液细胞外囊泡,特别是尿液外泌体,可能是一种有效的早期诊断和治疗糖尿病肾病的生物标记物。此外, 尿液细胞外囊泡已被作为糖尿病肾病的治疗靶标。
结论:
尿液细胞外囊泡中的蛋白质和RNA是诊断和治疗糖尿病肾病的潜在的生物标志物。
INTRODUCTION
Diabetic kidney disease (DKD) occurs in 20–40% of all patients with diabetes mellitus (DM).[1] It has become one of the major causes of chronic kidney disease (CKD) and end-stage renal disease (ESRD) worldwide. The mortality rate for DM has increased by 32.1% which led to 1.5 million deaths from 2005 to 2015.[2] According to the American Diabetes Association, DKD was defined as an estimated glomerular filtration rate (eGFR) of <60 ml·min−1·1.73 m−2 or detection of albuminuria in patients with DM.[3] For practical purposes, albuminuria was defined as a spot urinary albumin-to-creatinine ratio/albumin-to-creatinine ratio (UACR/ACR) higher than 30 mg/g. However, there are some limitations of using UACR and eGFR for diagnosing DKD. Recent studies suggested that microalbuminuria might not be a precise predictor for the risk of DKD due to considerable daily variation in albuminuria.[4] In addition, some conditions, including exercise within 24 h, high-protein diet, infection, fever, high blood pressure, urinary tract infection, and congestive heart failure, might result in falsely elevated UACR without any kidney damages.[3] It is also well-known that some diabetic patients could develop renal failure without preexisting albuminuria.[5] Given increased prevalence and significant socioeconomic burden caused by DKD, the noninvasive and more accurate early diagnostic biomarkers will be needed to improve the quality of patient life and reduce its impact on healthcare.
Urinary extracellular vesicles (uEVs), a potential noninvasive biomarker for early diagnosis and therapy of DKD, can help us understand the pathophysiological mechanisms leading to renal damage. EVs are vesicular structures enclosed by phospholipid bilayer and containing protein and nucleic acids.[6,7] The uEVs have been involved in the pathogenesis of acute kidney injury and CKD, including renal fibrosis, ESRD, glomerular diseases, and DKD.[8] This review focused on the application of uEVs for early diagnosis and treatment of DKD through a comprehensive review of the medical literature.
CHARACTERISTIC OF EXTRACELLULAR VESICLES
Generalization of extracellular vesicles
EVs were first discovered in sheep reticulocytes by Pan and Johnstone in 1983[9] and were named as “exosomes” in 1987.[10] One of the main criteria to define EVs is that they are isolated from the extracellular fluid.[11] EVs can be detected in human blood,[12] urine,[13] saliva,[14] bile,[15] cerebrospinal fluid,[16] breast milk,[17] amniotic fluid,[18] seminal fluid,[19] prostatic secretions,[20] ascites,[21] and pleural fluid.[22,23] There are three types of EVs: exosomes, microvesicles (MVs), and apoptotic bodies (ABs), and their diameters generally range from 40 to 150 nm, 100–1000 nm, and 50 nm − 5 μm, respectively.[20,21,22] Recently, newer types of EVs named as multivesicular spheres or pheresomes were found, due to its new mechanism of shedding from a spherical membrane structure.[24]
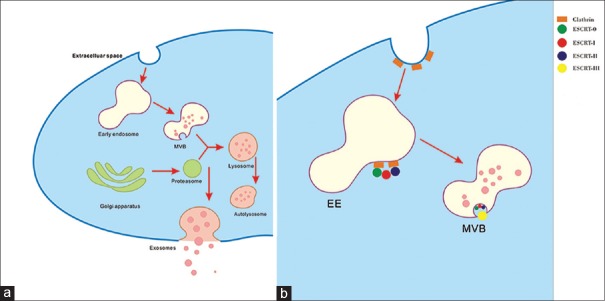
The biogenesis and release of exosomes can be roughly divided into two steps: the formation of multivesicular bodies (MVBs) and their fusion with the plasma membrane [Figure 1].[25] The endocytic vesicles mature to early endosomes and then the late endosomes by inward budding of the plasma membrane.[26] The late endosomes turn into MVBs by accumulating the intraluminal vesicles (ILVs) which consist of endosomal sorting complex required for transport, proteins, lipids, and RNAs.[23] Thereafter, the MVBs fuse with the plasma membrane and release the ILVs, which are called exosomes.[13] MVs are released by the outward budding of the plasma membrane or from the endosomal compartments.[27] ABs are released after cells undergo apoptosis.
Figure 1.
Biogenesis and secretion of exosomes: multivesicular body formation (a) and intraluminal vesicles formation (b) based on ESCRT complex involvement. ESCRT: Endosomal sorting complex required for transport; EE: Early endosome; MVB: Multivesicular body.
The mechanisms for uptaking EVs by target cells include clathrin-dependent[28] and clathrin-independent pinocytosis,[29] caveolin-mediated endocytosis,[30] and phagocytosis.[31] Heparin can block the communication between donor and recipient cells through EVs.[32]
EVs contain a variety of proteins, RNAs, and other substances. Moreover, most of these biomarkers are associated with kidney and genitourinary diseases.[33] A research had demonstrated that many mRNAs in the MVs were related to cell migration, angiogenesis, cell proliferation, immune response, and histone modification.[34] MVs contain varieties of angiogenic proteins, such as angiogenin and FGF which mediate the angiogenesis and calcification of vascular smooth muscle cells.[34,35] Besides, EVs can avoid complement-mediated lysis by expression of CD55 and CD59[36] and participate in antigen presentation between different cells of the immune system.[6] In addition, EVs are also involved in tumor metastasis. A study suggested that MVs derived from activated platelets could induce the metastasis of lung cancer.[37] Exosomes derived from tumor are an origin of tumor rejection antigens for cytotoxic T lymphocyte cross-priming.[38] Moreover, tumor MVs can deliver the genetic information and signaling proteins to surrounding cells.[34]
Isolation of extracellular vesicles
By far, there are no standardized technologies to isolate and purify EVs because of the complexity and variation of the sample composition. Therefore, it is difficult to compare EVs isolated by different techniques. As a result, development of a standardized, reliable, and efficient isolation method is necessary to ensure their appropriate clinical applications as well as downstream studies in genomics, proteomics of EVs.
The conventional isolation techniques include ultracentrifugation-based separation, size-based separation, and microfluidic separation. Based on the size and density of exosomes, we can collect exosomes by ultracentrifugation at 20,000 ×g. Ultracentrifugation is well known for differential centrifugation based on the size and density of the samples. However, this technique has some disadvantages such as time consuming, low yield, and low purification quality. A study reported that about 40% of the vesicles got lost in the supernatant after the differential centrifugation.[39] Sucrose gradient centrifugation (SGC) based on the different flotation densities could improve the purity by reducing interference of the amount of Tamm-Horsfall protein in urine.[40] SGC also has shortcomings since it cannot separate exosomes from viruses or MVs due to the similarity in buoyant density.[41]
Ultrafiltration is one of the most popular techniques for size-based separation. Ultrafiltration is not only faster than ultracentrifugation but also does not require special equipment.[42] However, this technique might cause deformation or even rupture of the EVs, which would affect the results of the following researches.[43] Another size-based separation is size exclusion chromatography (SEC). Some studies suggested that using SEC to isolate EVs could retain more intact biophysical properties, but the efficiency of this technique is low and approximately below 1%.[44] The third method is to use the nanomembrane concentrator. A research used a nanomembrane concentrator to enrich exosomes from urine by centrifugation at 3000 ×g for 10–30 min.[45] Despite the rapid development and advances in filtration technologies, there are no uniform standards for the membrane pore size, the order of the centrifugation steps, and no studies regarding the amount of vesicles lost in the membrane holes. One study demonstrated that hydrostatic filtration dialysis was a method with more advantages than the other methods, given that there is no need to buy expensive equipment and it is high efficiency.[39] This method mainly uses a simple device to form hydrostatic pressure in the dialysis membrane with relative molecular mass of 1,000,000; then, the liquid and soluble proteins are filtered out of the dialysis membrane, and the EVs are enriched in the dialysis membrane.[39]
A variety of commercial kits have been developed based on microfluidics. Acoustic nano-filter system separates EVs from other contents of the samples using a microfluidic device to collect the EVs. This method is highly efficient and can produce about 80% of the exosomes and more than 90% of the larger MVs.[46] In addition, acoustic filtering is faster than the conventional methods, and the acoustic damage to the ECS is minimal.[47] Davies et al.[48] had shown that direct-current electrophoresis can be employed as an alternative driving force to enhance purity in the microfluidic device. Wang et al.[49] have developed a porous silicon nanowire-on-micropillar structure based on the size difference between exosomes and other EVs as well as cellular debris. Kanwar et al.[50] showed a microfluidic-based platform named “ExoChip” in their study which has been used to isolate specific exosomes. They demonstrated the ability of ExoChip to recover exosomes with intact RNA enabling profiling of exosomal-microRNAs (miRNAs) through openarray analysis, which had potential applications in biomarker discovery. However, their findings encourage conducting a detailed prospective trial with a large-sample population and performing detailed molecular analysis.[50] To our knowledge, the most recently developed method is to integrate acoustics and microfluidics techniques to isolate exosomes or other types of EVs in an automated fashion.[51] Studies have shown that this method requires 100 ml of undiluted blood samples and only takes <25 min to complete the separation and isolation in contrast to 24 h by ultracentrifugation.[51] The researchers concluded that this technology generally did not change the biological or physical characteristics of the exosomes, had much less time consuming with high yield, and increased uniformity, compared to the conventional separation technologies.[51] They anticipated that this method would improve the quality and efficiency of isolation of exosomes and other EVs, hence promoting its clinical applications.[51]
However, up to date, there is no “gold standard” technology to separate and/or purify EVs. The most effective method may depend on the specific scientific issues required by and on the downstream applications used.[11] The International Society for Extracellular Vesicles (ISEV) recommended that researchers should describe the detailed methodology used for EV isolation and describe the characteristics of EVs in publication in order to let the researchers interpret and replicate the results.[11] The ISEV also recommended characterizing EVs at both population and single-vesicle levels.[11]
Urinary extracellular vesicles in kidneys
The uEVs are secreted by almost all cell types in kidneys such as glomerular epithelial cells, glomerular podocytes, tubular epithelial cells, and collecting duct cells.[13,52,53,54] In general, the circulating EVs cannot pass through the glomerulus and reach the urine under physiological conditions.[13] Gudehithlu et al.[55] found that the measurement of gelatinase in the urinary exosomes was more accurate during the progression of the diabetic nephropathy than the measurement of the whole urine gelatinase. Compared to the whole urine samples, proteins in the urinary exosomes were correlated better with the underlying protein changes in the kidney. They also observed that in humans, some inflammatory markers, such as ceruloplasmin levels, were elevated in the urinary exosomes much earlier than microalbuminuria in diabetic nephropathy.[55] Therefore, the contents in uEVs were more likely to reveal the overall kidney health.[56] Exosomes were first isolated by Pisitkun et al.[13] in urine in 2004. Subsequently, the larger vesicles were also found in urine, so uEV was a more appropriate name. The uptake of uEVs by renal collecting duct cells is regulated by vasopressin.[57] The uEVs contain a variety of proteins and RNAs and participate in the physiological and pathological processes of the kidney. UEVs transmit signals between neighboring cells, thus affecting the renal physiological functions.[17,53] Pisitkun et al.[13] found that several proteins were associated with certain renal diseases. For example, polycystin-1 and aquaporin (AQP)-2 are thought to be associated with polycystic kidney disease and autosomal recessive nephrogenic diabetes insipidus, respectively. Hogan et al.[40] showed similar findings on polycystin-1 by transmission electron microscopy images. Puhka et al.[58] examined the transcriptome of uEVs via RNA sequencing and found that uEVs were overexpressed in cellular metabolism, vesicle trafficking, and mitochondrial and ribosome functions. As a result, uEVs, especially urinary exosomes, might be useful biomarkers for early diagnosis and treatment to DKD.
UTILITY OF URINARY EXTRACELLULAR VESICLES FOR DIAGNOSING DIABETIC KIDNEY DISEASE
UEVs have become a hot topic recently because the urine is easy to obtain and it is noninvasive. Many in vitro cell experiments, animal experiments, and clinical trials have been carried out and showed that the proteins and miRNAs detected in uEVs could be a promising marker for diagnosing DKD, although the widespread clinical application requires further validation.
Proteins in urinary extracellular vesicles for diagnosing
An experiment showed that the contents of C-megalin in uEVs increased during the progression of albuminuria in DM patients.[59] Hence, C-megalin in uEVs might be associated with the development and progression of DKD. Using three different mouse models with Type 1 diabetes, Burger et al.[60] found that there was 5-fold increase in urine MVs produced by podocytes during the early stages of diabetic renal injury, which preceded the presence of albuminuria. Rossi et al.[5] analyzed the AQP2 and AQP5 (uAQP5 and uAQP2) excreted in urine in 35 patients: 12 diabetic patients with normoalbuminuria and normal renal function, 11 with proteinuria nondiabetic nephropathy, and 12 with histological diagnosis and classification of DKD. Interestingly, the enzyme-linked immunosorbent assay (ELISA) and Western blot analysis independently showed that uAQP5 was significantly increased in DKD patients. Furthermore, linear regression analysis showed a positive correlation between uAQP5 and the histological diagnosis of DKD.[5] The studies of uAQP2 showed similar results. Although the sample size was somewhat small, these studies suggested that AQP5 and AQP2 could be used as new noninvasive biomarkers for early diagnosis of DKD.[5] A study from Musante et al.[61] showed that the profiles of comprehensive protease in uEVs were significantly changed in DKD. They analyzed the proteases and protease inhibitors in uEVs isolated by hydrostatic dialysis and demonstrated an increase of myeloblast and its natural inhibitor in the normal- and microalbuminuria groups. Zubiri et al.[62] identified 352 different proteins in human urinary exosomes using liquid chromatography-mass spectrometry. Among these proteins, three of them showed different levels in DKD patients compared to the patients without DKD. These three proteins are α-microglobulin/bikunin precursor, histone-lysine N-methyltransferase (MLL3), and voltage-dependent anion-selective channel protein 1 (VDAC1). In addition, they did similar study using a rat model with early DKD and found that regucalcin protein, also known as senescence marker protein-30, was strongly downregulated in exosomes isolated from urine of these animal models. Later on, these results were confirmed in a pilot study using human samples.[63] Wilms tumor-1 protein (WT1), a protein secreted by renal epithelial cells and detected in uEVs, is thought to be related to podocyte injury. A study developed by Kalani et al.[64] suggested that the level of WT1 protein in uEVs was associated with the increase of ACR and serum creatinine as well as decline in eGFR. Although these findings suggested that the level of WT1 in uEVs might be relevant to the renal functions, some other studies revealed that WT1 in uEVs might be only a marker of podocyte injury but not a specific marker seen in DKD.[52,65] Sun et al.[66] have done a study to evaluate the relationship between DKD and dipeptidyl peptidase-IV (DPP IV) protein detected in urinary MVs. Total 127 diabetic patients and 34 healthy individuals were included in the study. Among the diabetic patients, 43 patients had normoalbuminuria, 50 patients had microalbuminuria, and 34 patients had macroalbuminuria. They measured DPP IV protein levels by ELISA and Western blot analysis and found that the levels of DPP IV protein in urinary MVs of DKD patients were higher than controls, and positively correlated with UACR.
These studies suggested that a variety of proteins presented in the uEVs might be associated with the development and progression of DKD, while some proteins could be potential noninvasive biomarkers for early diagnosis of incipient DKD. However, it is worth noting that some proteins are not specific for DKD and may also be associated with other diseases. Different uEVs isolation methods may lead to different results because of the interference of other proteins. Therefore, additional studies are needed to further verify the diagnostic utility of proteins in uEVs.
MicroRNA in urinary extracellular vesicles for diagnosing diabetic kidney disease
MiRNAs are a group of noncoding RNAs, involved in a variety of physiological and pathological processes, such as apoptosis, proliferation, regulation of immune responses, insulin secretion, and cell differentiation.[67] Miranda et al.[56] found that MVs isolated from human urine had an RNA integrity profile similar to that of kidney tissue. Meanwhile, they developed a rapid and reliable method to isolate nucleic acids from urinary MVs. The miRNA predominantly exists in exosomes in a stable form in uEVs. Kidney tissue contains miR-192, miR-194, and miR-215, and they play a critical role in the pathogenesis of DKD.[68] Jia et al.[69] analyzed uEV miRNA expression by real-time PCR in 80 patients diagnosed with Type 2 diabetes (30 normoalbuminuria, 30 microalbuminuria, and 20 macroalbuminuria) and 10 healthy controls. The receiver operating characteristic curve showed that miR-192 was better than miR-194 and miR-215 in identifying DKD in the patients with normoalbuminuria or microalbuminuria, which indicated that uEVs miR-192 might be able to diagnose DKD earlier. Eissa et al.[70] examined the miRNAs expression in urine exosomes from 210 participants by quantitative reverse transcription-polymerase chain reaction (PCR) and found that urinary exosome miR-133b, miR-342, and miR-30a were upregulated in DKD patients compared to normal controls. Another research also demonstrated that miR-15b, miR-34a, and miR-636 were highly expressed in urinary exosomes of DKD measured by real-time PCR.[71] Delić et al.[72] described a differential expression of 16 miRNAs in urinary exosomes and concluded that, compared to healthy controls, the expression of 14 miRNAs (miR-320c, miR-6068, miR-1234-5p, miR-6133, miR-4270, miR-4739, miR-371b-5p, miR-638, miR-572, miR-1227-5p, miR-6126, miR-1915-5p, miR-4778-5p, and miR-2861) was increased whereas the expression of 2 miRNAs (miR-30d-5p and miR-30e-5p) was declined in Type 2 diabetes-induced DKD. Barutta et al.[73] found that miR-130a and miR-145 were increased, while miR-155 and miR-424 were declined in urinary exosomes from DM patients with microalbuminuria, but the changes were not detected in patients with normoalbuminuria. Similarly, by testing miRNAs in urinary exosomes from diabetic rats, Mohan et al.[74] reported that the levels of miR 215 and miR 494 were significantly different in diabetic rats with severe renal pathology or higher glomerulosclerosis index compared to the diabetic rats with only moderate pathology.
More and more miRNAs in uEVs are identified and some of the corresponding gene sequences have been identified. Many studies have revealed their relevance to DKD. However, the clinical application of these results is still premature. We anticipate that more novel miRNAs related to DKD will be discovered and more specific biomarkers and commercial diagnostic kits can be used in clinical practice in the near future.
ROLES OF URINARY EXTRACELLULAR VESICLES IN TREATMENT OF DIABETIC KIDNEY DISEASE
As delivery systems for therapeutic agents
EVs can serve as transporters for therapeutic drugs due to their ability to transport proteins and nucleic acids among different cells. Some studies suggested that chemotherapeutic agents loaded in exosomes could be delivered to malignant cells.[75] Up to date, Phase I and II clinical trials have also shown that dendritic exosomes could be used to treat malignancies.[75] However, there are some issues that have not been solved yet. First, the specific molecular mechanisms of the production, secretion, and uptake of EVs are still unclear.[74] Second, the techniques to incorporate therapeutic agents into the EVs and deliver them to the target cells have not been studied very well.[76] These challenges have hindered the clinical application of EVs as a drug delivery system. Hence, a large number of studies and clinical trials are warranted before uEVs can be used as an effective delivery system.
As potential therapeutic targets
Exosomes from urine and other biological fluids can serve as therapeutic targets for the treatment of renal diseases.[77] Glomerular mesangial cell (GMC) activation was thought to be associated with DKD.[78] It is well known that transforming growth factor beta 1 (TGF-β1) can induce GMCs activation and proliferation, ultimately lead to renal fibrosis.[78,79] Wu et al.[80] demonstrated that TGF-β1-containing exosomes from high glucose-treated glomerular endothelial cells (GECs) could activate GMCs and promote renal fibrosis through the TGF-β1/Smad3 signaling pathway. Thus, inhibition of intercellular transmission of TGF-β1-containing exosomes from GECs to GMCs might be a novel target for the prevention and treatment of DKD. A recent study identified that the transcription factor sterol regulatory element-binding protein 1 (SREBP-1) was a novel regulator of TGF-β1 receptor I expression.[81] As a result, inhibition of SREBP-1 in vivo could be a practical therapeutic strategy for DKD. Sun et al.[82] reported that CD63-positive exosome levels were significantly higher in patients with normoalbuminuria than patients with microalbuminuria and only decreased markedly after therapy with alpha-lipoic acid (α-LA). Therefore, CD63-positive exosomes might also be used as a potential therapeutic indicator for DKD. Kim et al.[83] found that activating transcription factor-3 in the uEVs might be an important regulator by enhancing extracellular matrix proliferation and macrophage infiltration, which revealed a new aspect of the therapeutic mechanisms of DKD.
Till now, the numbers of studies focused on uEVs as a therapeutic target were significantly less than those which have been done for diagnosing incipient DKD using uEVs. This could be due to the mechanisms that uEVS involved in the pathogenesis of DKD are still not very clear. Therefore, more studies are needed to further understand the roles of uEVs in the pathogenesis of DKD.
CONCLUSIONS
EVs are involved in a variety of physiological and pathological processes. Understanding the pathophysiological features, production, secretion, and uptake of EVs can further help us comprehend their roles in the development and progression of renal diseases. Successful and efficient isolation and identification of EVs is urgent. However, there is still no standardized and widely accepted method of isolation available by far. Future studies focusing on the development and improvement of EVs isolation and identification technologies are in need. The detailed pathophysiological mechanisms of uEVs in DKD need to be further addressed. The multicenter and prospective studies of early diagnosis and treatment of DKD by uEVs are critical to understand uEVs in depth and subsequently utilize them in clinical practice. In summary, proteins and nucleic acids in UEVs represent promising biomarker for the early diagnosis and treatment of DKD.
Financial support and sponsorship
This study was supported by grants from the Guangdong Provincial Science and Technology Project (No. 2014A020212662), the Science and Technology Planning Project of Southern Medical University (No. CX2016N018), the Science and Technology Planning Project of Tianhe District, Guangzhou city (No. 201704KW011), The Natural Science Foundation of Guangdong Province (2016A030313559), and The South Wisdom Valley Innovative Research Team Program (No. CXTD-004, 2014).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
REFERENCES
- 1.Kim SS, Kim JH, Kim IJ. Current challenges in diabetic nephropathy: Early diagnosis and ways to improve outcomes. Endocrinol Metab (Seoul) 2016;31:245–53. doi: 10.3803/EnM.2016.31.2.245. doi: 10.3803/EnM.2016.31.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–544. doi: 10.1016/S0140-6736(16)31012-1. doi: 10.1016/s0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Erratum Microvascular complications and foot care. Sec 10. In Standards of medical care in diabetes-2017 Diabetes care-2017. 2017;40(Suppl 1):S88–S98. doi: 10.2337/dc17-er07c. doi: 10.2337/dc17.er07c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, et al. Diabetic kidney disease: A report from an ADA consensus conference. Diabetes Care. 2014;37:2864–83. doi: 10.2337/dc14-1296. doi: 10.2337/dc14-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossi L, Nicoletti MC, Carmosino M, Mastrofrancesco L, Di Franco A, Indrio F, et al. Urinary excretion of kidney aquaporins as possible diagnostic biomarker of diabetic nephropathy. J Diabetes Res. 2017;2017:4360357. doi: 10.1155/2017/4360357. doi: 10.1155/2017/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–72. doi: 10.1084/jem.183.3.1161. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Théry C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang W, Zhou X, Zhang H, Yao Q, Liu Y, Dong Z, et al. Extracellular vesicles in diagnosis and therapy of kidney diseases. Am J Physiol Renal Physiol. 2016;311:F844–51. doi: 10.1152/ajprenal.00429.2016. doi: 10.1152/ajprenal.00429.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes In vitro: Selective externalization of the receptor. Cell. 1983;33:967–78. doi: 10.1016/0092-8674(83)90040-5. doi: 10.1016/0092-8674(83) 90040-5. [DOI] [PubMed] [Google Scholar]
- 10.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–20. [PubMed] [Google Scholar]
- 11.Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. doi: 10.3402/jev.v3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17:879–87. doi: 10.1093/intimm/dxh267. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 13.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A. 2004;101:13368–73. doi: 10.1073/pnas.0403453101. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogawa Y, Kanai-Azuma M, Akimoto Y, Kawakami H, Yanoshita R. Exosome-like vesicles with dipeptidyl peptidase IV in human saliva. Biol Pharm Bull. 2008;31:1059–62. doi: 10.1248/bpb.31.1059. doi: 10.1248/bpb.31.1059. [DOI] [PubMed] [Google Scholar]
- 15.Li L, Piontek KB, Kumbhari V, Ishida M, Selaru FM. Isolation and profiling of MicroRNA-containing exosomes from human bile. J Vis Exp. 2016;112:e54036. doi: 10.3791/54036. doi: 10.3791/54036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Street JM, Barran PE, Mackay CL, Weidt S, Balmforth C, Walsh TS, et al. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J Transl Med. 2012;10:5. doi: 10.1186/1479-5876-10-5. doi: 10.1186/1479-5876-10- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Admyre C, Johansson SM, Qazi KR, Filén JJ, Lahesmaa R, Norman M, et al. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179:1969–78. doi: 10.4049/jimmunol.179.3.1969. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- 18.Asea A, Jean-Pierre C, Kaur P, Rao P, Linhares IM, Skupski D, et al. Heat shock protein-containing exosomes in mid-trimester amniotic fluids. J Reprod Immunol. 2008;79:12–7. doi: 10.1016/j.jri.2008.06.001. doi: 10.1016/j.jri.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Poliakov A, Spilman M, Dokland T, Amling CL, Mobley JA. Structural heterogeneity and protein composition of exosome-like vesicles (prostasomes) in human semen. Prostate. 2009;69:159–67. doi: 10.1002/pros.20860. doi: 10.1002/pros.20860. [DOI] [PubMed] [Google Scholar]
- 20.Aalberts M, Stout TA, Stoorvogel W. Prostasomes: Extracellular vesicles from the prostate. Reproduction. 2014;147:R1–14. doi: 10.1530/REP-13-0358. doi: 10.1530/rep-13-0358. [DOI] [PubMed] [Google Scholar]
- 21.Shender VO, Pavlyukov MS, Ziganshin RH, Arapidi GP, Kovalchuk SI, Anikanov NA, et al. Proteome-metabolome profiling of ovarian cancer ascites reveals novel components involved in intercellular communication. Mol Cell Proteomics. 2014;13:3558–71. doi: 10.1074/mcp.M114.041194. doi: 10.1074/mcp.M114.041194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bard MP, Hegmans JP, Hemmes A, Luider TM, Willemsen R, Severijnen LA, et al. Proteomic analysis of exosomes isolated from human malignant pleural effusions. Am J Respir Cell Mol Biol. 2004;31:114–21. doi: 10.1165/rcmb.2003-0238OC. doi: 10.1165/rcmb.2003-0238OC. [DOI] [PubMed] [Google Scholar]
- 23.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89. doi: 10.1146/annurev-cellbio-101512-122326. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 24.Junquera C, Castiella T, Muñoz G, Fernández-Pacheco R, Luesma MJ, Monzón M, et al. Biogenesis of a new type of extracellular vesicles in gastrointestinal stromal tumors: Ultrastructural profiles of spheresomes. Histochem Cell Biol. 2016;146:557–67. doi: 10.1007/s00418-016-1460-5. doi: 10.1007/s00418-016-1460-5. [DOI] [PubMed] [Google Scholar]
- 25.Musante L, Tataruch DE, Holthofer H. Use and isolation of urinary exosomes as biomarkers for diabetic nephropathy. J Diabetes Res. 2014;5:149. doi: 10.3389/fendo.2014.00149. doi: 10.3389/fendo.2014.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoorvogel W, Strous GJ, Geuze HJ, Oorschot V, Schwartz AL. Late endosomes derive from early endosomes by maturation. Cell. 1991;65:417–27. doi: 10.1016/0092-8674(91)90459-c. doi: 10.1016/0092-8674(91)90459-C. [DOI] [PubMed] [Google Scholar]
- 27.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838–48. doi: 10.1038/ki.2010.278. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 28.Tian T, Zhu YL, Zhou YY, Liang GF, Wang YY, Hu FH, et al. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J Biol Chem. 2014;289:22258–67. doi: 10.1074/jbc.M114.588046. doi: 10.1074/jbc.M114.588046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Damke H, Baba T, van der Bliek AM, Schmid SL. Clathrin-independent pinocytosis is induced in cells overexpressing a temperature-sensitive mutant of dynamin. J Cell Biol. 1995;131:69–80. doi: 10.1083/jcb.131.1.69. doi: 10.1083/jcb.131.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shankar J, Boscher C, Nabi IR. Caveolin-1, galectin-3 and lipid raft domains in cancer cell signalling. Essays Biochem. 2015;57:189–201. doi: 10.1042/bse0570189. doi: 10.1042/bse0570189. [DOI] [PubMed] [Google Scholar]
- 31.Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF, et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010;11:675–87. doi: 10.1111/j.1600-0854.2010.01041.x. doi: 10.1111/j.1600-0854.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- 32.Atai NA, Balaj L, van Veen H, Breakefield XO, Jarzyna PA, Van Noorden CJ, et al. Heparin blocks transfer of extracellular vesicles between donor and recipient cells. J Neurooncol. 2013;115:343–51. doi: 10.1007/s11060-013-1235-y. doi: 10.1007/s11060-013-1235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Street JM, Koritzinsky EH, Glispie DM, Star RA, Yuen PS. Urine exosomes: An emerging trove of biomarkers. Adv Clin Chem. 2017;78:103–22. doi: 10.1016/bs.acc.2016.07.003. doi: 10.1016/bs.acc.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–6. doi: 10.1038/ncb1800. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kapustin AN, Chatrou ML, Drozdov I, Zheng Y, Davidson SM, Soong D, et al. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ Res. 2015;116:1312–23. doi: 10.1161/CIRCRESAHA.116.305012. doi: 10.1161/circresaha.116.305012. [DOI] [PubMed] [Google Scholar]
- 36.Clayton A, Harris CL, Court J, Mason MD, Morgan BP. Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. Eur J Immunol. 2003;33:522–31. doi: 10.1002/immu.200310028. doi: 10.1002/immu.200310028. [DOI] [PubMed] [Google Scholar]
- 37.Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, et al. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005;113:752–60. doi: 10.1002/ijc.20657. doi: 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- 38.Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 39.Musante L, Tataruch-Weinert D, Kerjaschki D, Henry M, Meleady P, Holthofer H, et al. Residual urinary extracellular vesicles in ultracentrifugation supernatants after hydrostatic filtration dialysis enrichment. J Extracell Vesicles. 2017;6:1267896. doi: 10.1080/20013078.2016.1267896. doi: 10.1080/20013078.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hogan MC, Manganelli L, Woollard JR, Masyuk AI, Masyuk TV, Tammachote R, et al. Characterization of PKD protein-positive exosome-like vesicles. J Am Soc Nephrol. 2009;20:278–88. doi: 10.1681/ASN.2008060564. doi: 10.1681/ASN.2008060564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AM, et al. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. 2012;56:293–304. doi: 10.1016/j.ymeth.2012.01.002. doi: 10.1016/j.ymeth.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Chinello C, Musante L, Cazzaniga M, Tataruch D, Calzaferri G, et al. Intraluminal proteome and peptidome of human urinary extracellular vesicles. Proteomics Clin Appl. 2015;9:568–73. doi: 10.1002/prca.201400085. doi: 10.1002/prca.201400085. [DOI] [PubMed] [Google Scholar]
- 43.Zeringer E, Barta T, Li M, Vlassov AV. Strategies for isolation of exosomes. Cold Spring Harb Protoc. 2015;2015:319–23. doi: 10.1101/pdb.top074476. doi: 10.1101/pdb.top074476. [DOI] [PubMed] [Google Scholar]
- 44.Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405. doi: 10.1016/j.jconrel.2015.07.030. doi: 10.1016/j.jconrel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nordin JZ, Lee Y, Vader P, Mäger I, Johansson HJ, Heusermann W, et al. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomedicine. 2015;11:879–83. doi: 10.1016/j.nano.2015.01.003. doi: 10.1016/j.nano.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Cheruvanky A, Zhou H, Pisitkun T, Kopp JB, Knepper MA, Yuen PS, et al. Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. Am J Physiol Renal Physiol. 2007;292:F1657–61. doi: 10.1152/ajprenal.00434.2006. doi: 10.1152/ajprenal.00434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee K, Shao H, Weissleder R, Lee H. Acoustic purification of extracellular microvesicles. ACS Nano. 2015;9:2321–7. doi: 10.1021/nn506538f. doi: 10.1021/nn506538f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davies RT, Kim J, Jang SC, Choi EJ, Gho YS, Park J, et al. Microfluidic filtration system to isolate extracellular vesicles from blood. Lab Chip. 2012;12:5202–10. doi: 10.1039/c2lc41006k. doi: 10.1039/c2lc41006k. [DOI] [PubMed] [Google Scholar]
- 49.Wang Z, Wu HJ, Fine D, Schmulen J, Hu Y, Godin B, et al. Ciliated micropillars for the microfluidic-based isolation of nanoscale lipid vesicles. Lab Chip. 2013;13:2879–82. doi: 10.1039/c3lc41343h. doi: 10.1039/c3lc41343h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanwar SS, Dunlay CJ, Simeone DM, Nagrath S. Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip. 2014;14:1891–900. doi: 10.1039/c4lc00136b. doi: 10.1039/c4lc00136b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu M, Ouyang Y, Wang Z, Zhang R, Huang PH, Chen C, et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc Natl Acad Sci U S A. 2017;114:10584–9. doi: 10.1073/pnas.1709210114. doi: 10.1073/pnas.1709210114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou H, Cheruvanky A, Hu X, Matsumoto T, Hiramatsu N, Cho ME, et al. Urinary exosomal transcription factors, a new class of biomarkers for renal disease. Kidney Int. 2008;74:613–21. doi: 10.1038/ki.2008.206. doi: 10.1038/ki.2008.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dear JW, Street JM, Bailey MA. Urinary exosomes: A reservoir for biomarker discovery and potential mediators of intrarenal signalling. Proteomics. 2013;13:1572–80. doi: 10.1002/pmic.201200285. doi: 10.1002/pmic.201200285. [DOI] [PubMed] [Google Scholar]
- 54.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–67. doi: 10.1681/ASN.2008070798. doi: 10.1681/asn.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gudehithlu KP, Garcia-Gomez I, Vernik J, Brecklin C, Kraus M, Cimbaluk DJ, et al. In diabetic kidney disease urinary exosomes better represent kidney specific protein alterations than whole urine. Am J Nephrol. 2015;42:418–24. doi: 10.1159/000443539. doi: 10.1159/000443539. [DOI] [PubMed] [Google Scholar]
- 56.Miranda KC, Bond DT, McKee M, Skog J, Păunescu TG, Da Silva N, et al. Nucleic acids within urinary exosomes/microvesicles are potential biomarkers for renal disease. Kidney Int. 2010;78:191–9. doi: 10.1038/ki.2010.106. doi: 10.1038/ki.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oosthuyzen W, Scullion KM, Ivy JR, Morrison EE, Hunter RW, Starkey Lewis PJ, et al. Vasopressin regulates extracellular vesicle uptake by kidney collecting duct cells. J Am Soc Nephrol. 2016;27:3345–55. doi: 10.1681/ASN.2015050568. doi: 10.1681/asn.2015050568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Puhka M, Dwivedi O, Forsblom C, Valo E, Barreiro K, Holthöfer H, et al. Urine extracellular vesicles transcriptome in diabetic kidney disease. J Extracell Vesicles. 2017;6:53. doi: 10.1002/jev2.12038. doi: 10.1080/20013078.2017.1310414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De S, Kuwahara S, Hosojima M, Ishikawa T, Kaseda R, Sarkar P, et al. Exocytosis-mediated urinary full-length megalin excretion is linked with the pathogenesis of diabetic nephropathy. Diabetes. 2017;66:1391–404. doi: 10.2337/db16-1031. doi: 10.2337/db16-1031. [DOI] [PubMed] [Google Scholar]
- 60.Burger D, Thibodeau JF, Holterman CE, Burns KD, Touyz RM, Kennedy CR, et al. Urinary podocyte microparticles identify prealbuminuric diabetic glomerular injury. J Am Soc Nephrol. 2014;25:1401–7. doi: 10.1681/ASN.2013070763. doi: 10.1681/asn.2013070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Musante L, Tataruch D, Gu D, Liu X, Forsblom C, Groop PH, et al. Proteases and protease inhibitors of urinary extracellular vesicles in diabetic nephropathy. J Diabetes Res. 2015;2015:289734. doi: 10.1155/2015/289734. doi: 10.1155/2015/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zubiri I, Posada-Ayala M, Sanz-Maroto A, Calvo E, Martin-Lorenzo M, Gonzalez-Calero L, et al. Diabetic nephropathy induces changes in the proteome of human urinary exosomes as revealed by label-free comparative analysis. J Proteomics. 2014;96:92–102. doi: 10.1016/j.jprot.2013.10.037. doi: 10.1016/j.jprot.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 63.Zubiri I, Posada-Ayala M, Benito-Martin A, Maroto AS, Martin-Lorenzo M, Cannata-Ortiz P, et al. Kidney tissue proteomics reveals regucalcin downregulation in response to diabetic nephropathy with reflection in urinary exosomes. Transl Res. 2015;166:474–84e4. doi: 10.1016/j.trsl.2015.05.007. doi: 10.1016/j.trsl.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 64.Kalani A, Mohan A, Godbole MM, Bhatia E, Gupta A, Sharma RK, et al. Wilm's tumor-1 protein levels in urinary exosomes from diabetic patients with or without proteinuria. PLoS One. 2013;8:e60177. doi: 10.1371/journal.pone.0060177. doi: 10.1371/journal.pone.0060177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou H, Kajiyama H, Tsuji T, Hu X, Leelahavanichkul A, Vento S, et al. Urinary exosomal wilms' tumor-1 as a potential biomarker for podocyte injury. Am J Physiol Renal Physiol. 2013;305:F553–9. doi: 10.1152/ajprenal.00056.2013. doi: 10.1152/ajprenal.00056.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun AL, Deng JT, Guan GJ, Chen SH, Liu YT, Cheng J, et al. Dipeptidyl peptidase-IV is a potential molecular biomarker in diabetic kidney disease. Diab Vasc Dis Res. 2012;9:301–8. doi: 10.1177/1479164111434318. doi: 10.1177/1479164111434318. [DOI] [PubMed] [Google Scholar]
- 67.Chang TC, Mendell JT. MicroRNAs in vertebrate physiology and human disease. Annu Rev Genomics Hum Genet. 2007;8:215–39. doi: 10.1146/annurev.genom.8.080706.092351. doi: 10.1146/annurev.genom.8.080706.092351. [DOI] [PubMed] [Google Scholar]
- 68.Sun Y, Koo S, White N, Peralta E, Esau C, Dean NM, et al. Development of a micro-array to detect human and mouse microRNAs and characterization of expression in human organs. Nucleic Acids Res. 2004;32:e188. doi: 10.1093/nar/gnh186. doi: 10.1093/nar/gnh186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jia Y, Guan M, Zheng Z, Zhang Q, Tang C, Xu W, et al. MiRNAs in urine extracellular vesicles as predictors of early-stage diabetic nephropathy. J Diabetes Res. 2016;2016:7932765. doi: 10.1155/2016/7932765. doi: 10.1155/2016/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eissa S, Matboli M, Bekhet MM. Clinical verification of a novel urinary microRNA panal: 133b, -342 and -30 as biomarkers for diabetic nephropathy identified by bioinformatics analysis. Biomed Pharmacother. 2016;83:92–9. doi: 10.1016/j.biopha.2016.06.018. doi: 10.1016/j.biopha.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 71.Eissa S, Matboli M, Aboushahba R, Bekhet MM, Soliman Y. Urinary exosomal microRNA panel unravels novel biomarkers for diagnosis of type 2 diabetic kidney disease. J Diabetes Complications. 2016;30:1585–92. doi: 10.1016/j.jdiacomp.2016.07.012. doi: 10.1016/j.jdiacomp.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 72.Delić D, Eisele C, Schmid R, Baum P, Wiech F, Gerl M, et al. Urinary exosomal miRNA signature in type II diabetic nephropathy patients. PLoS One. 2016;11:e0150154. doi: 10.1371/journal.pone.0150154. doi: 10.1371/journal.pone.0150154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barutta F, Tricarico M, Corbelli A, Annaratone L, Pinach S, Grimaldi S, et al. Urinary exosomal microRNAs in incipient diabetic nephropathy. PLoS One. 2013;8:e73798. doi: 10.1371/journal.pone.0073798. doi: 10.1371/journal.pone.0073798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mohan A, Singh R, Garg D, Upadhyay A, Ghildiyal S, Tiwari S. Change in microRNA profile predicts diabetes associated renal pathology noninvasively. FASEB J. 2015;29 doi: 101096/fasebj291_supplement9594. [Google Scholar]
- 75.Karpman D, Ståhl AL, Arvidsson I. Extracellular vesicles in renal disease. Nat Rev Nephrol. 2017;13:545–62. doi: 10.1038/nrneph.2017.98. doi: 10.1038/nrneph.2017.98. [DOI] [PubMed] [Google Scholar]
- 76.Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–90. doi: 10.1016/j.biomaterials.2013.11.083. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 77.Merchant ML, Rood IM, Deegens JK, Klein JB. Isolation and characterization of urinary extracellular vesicles: Implications for biomarker discovery. Nat Rev Nephrol. 2017;13:731–49. doi: 10.1038/nrneph.2017.148. doi: 10.1038/nrneph.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ziyadeh FN. Mediators of diabetic renal disease: The case for tgf-beta as the major mediator. J Am Soc Nephrol. 2004;15(Suppl 1):S55–7. doi: 10.1097/01.asn.0000093460.24823.5b. doi: 10.1097/01.ASN.0000093460.24823.5B. [DOI] [PubMed] [Google Scholar]
- 79.Schnaper HW, Hayashida T, Hubchak SC, Poncelet AC. TGF-beta signal transduction and mesangial cell fibrogenesis. Am J Physiol Renal Physiol. 2003;284:F243–52. doi: 10.1152/ajprenal.00300.2002. doi: 10.1152/ajprenal.00300.2002. [DOI] [PubMed] [Google Scholar]
- 80.Wu XM, Gao YB, Cui FQ, Zhang N. Exosomes from high glucose-treated glomerular endothelial cells activate mesangial cells to promote renal fibrosis. Biol Open. 2016;5:484–91. doi: 10.1242/bio.015990. doi: 10.1242/bio.015990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van Krieken R, Chen G, Gao B, Read J, Al Saleh HA, Li R, et al. Sterol regulatory element binding protein (SREBP)-1 is a novel regulator of the transforming growth factor (TGF)-β receptor I (TβRI) through exosomal secretion. Cell Signal. 2017;29:158–67. doi: 10.1016/j.cellsig.2016.11.004. doi: 10.1016/j.cellsig.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 82.Sun H, Yao W, Tang Y, Zhuang W, Wu D, Huang S, et al. Urinary exosomes as a novel biomarker for evaluation of α-lipoic acid's protective effect in early diabetic nephropathy. J Clin Lab Anal. 2017;31:e22129. doi: 10.1002/jcla.22129. doi: 10.1002/jcla.22129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim J, Lee D, Park K, Kim G, Jeong E, Kim W. Activating transcription factor-3 plays as a novel potent regulator of renal fibrosis in streptozotocin-induced diabetic nephropathy model. Mol Biol Cell. 2013;24:3775. doi: 10.1091/mbc.E13-10-0584. [Google Scholar]