Fig. 5.
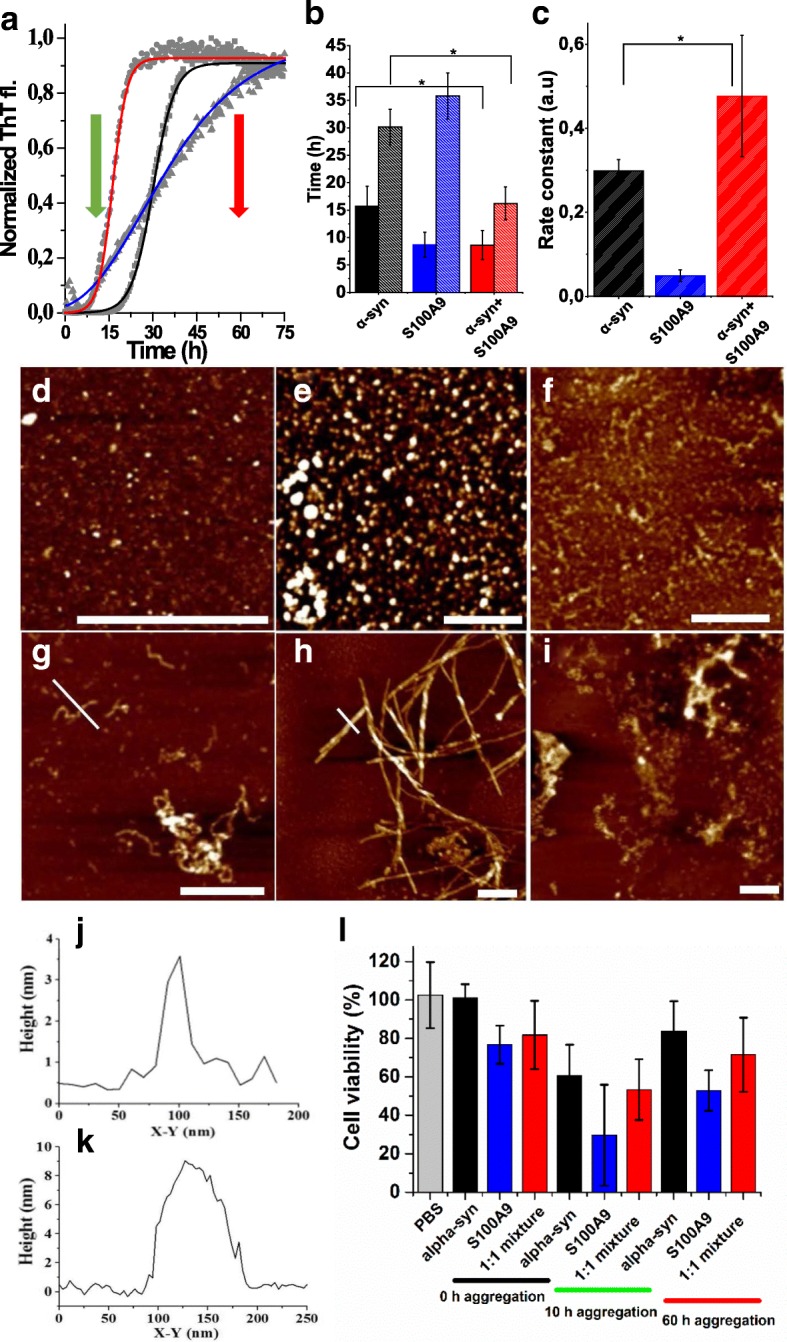
Amyloid co-aggregation and cytotoxicity of S100A9 and α-syn. a Normalized kinetic curves of amyloid formation monitored by ThT fluorescence and fitted by sigmoidal function for 70 μM α-syn (in black), 70 μM S100A9 (blue), and both proteins taken at equimolar ratio (red). Experimental data points are shown in gray. b Lag phase (dark bars) and midpoint of growth phase (light bars) of the amyloid formation kinetics derived from fitting. Protein samples are indicated under the x-axis and in the same color coding as in a. c Growth rate constant derived from fitting. Protein samples are indicated under the x-axis and in the same color coding as in a. Error bars represent SD. p ≤ 0.05 is indicated by *. d–i AFM height images of oligomeric/protofilament species of d S100A9, e α-syn, and f their equimolar mixture formed after 10 h incubation; amyloid fibrils of g S100A9, h α-syn, and i their co-aggregates at equimolar ratio of both proteins formed after 60 h incubation. Scale bars equal to 500 nm in all images. j, k AFM cross-sections of amyloid fibrils of S100A9 and α-syn, as shown by white cross-sections in g, h, respectively. l Viability of SH-SY5Y cells measured by WST-1 assay after 24 h co-incubation with α-syn and S100A9 species. Viability of cells treated with PBS alone is shown by gray bar; cells treated with 10 μM α-syn, 10 μM S100A9, or their equimolar mixture are shown by black, blue, and red bars, respectively. The durations of amyloid sample aggregation prior adding to cell culture, i.e., 0, 10, and 60 h, are indicated under the x-axis by black, green, and red bars, respectively; the oligomeric and fibrillar sample time collections are marked by red and green arrows, respectively, in the amyloid formation kinetics in a. Error bars represent SD of at least nine measurements. All protein samples were incubated in PBS, pH 7.4 and 37 °C
