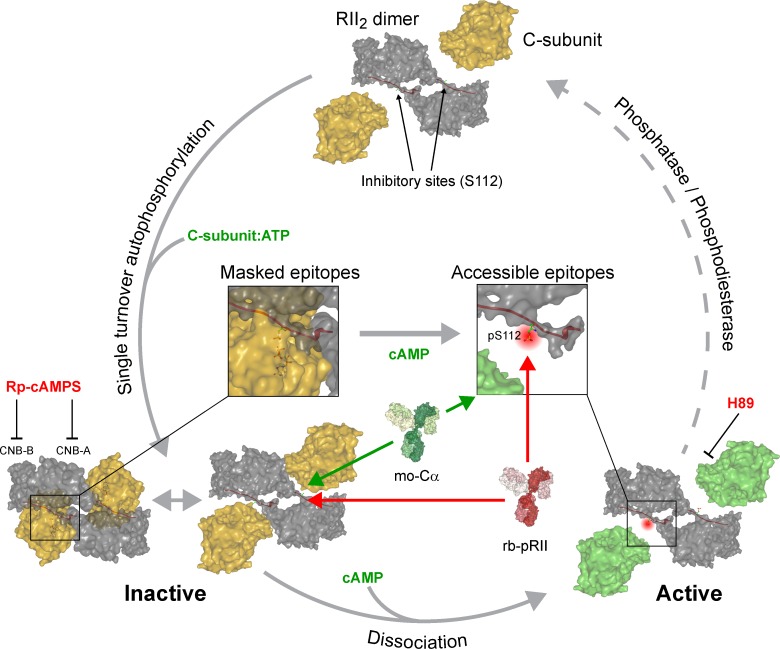
Figure 10.
Proposed model of PKA-II activation based on quantifying pRII and Cα signal intensities in intact sensory neurons. Our data support that the RII dimer associates with ATP-loaded C-subunits, resulting in a single-turnover autophosphorylation event (Zhang et al., 2015). Phosphorylated RII-subunits remain associated with C-subunits in the inactive holoenzyme. This complex is prone for conformational flexibility at the active site even in the absence of cAMP, making a fraction of RII inhibitor sites (pS112) as well as residues in the catalytic cleft of C-subunits accessible for antibodies. The presence of cAMP then induces full opening of holoenzymes, resulting in increased accessibility for antibodies recognizing pS112 of RII (red) or the glycine-rich loop of C-subunits (green). Reassociation of RII and C-subunits requires the concerted hydrolysis of cAMP by phosphodiesterases and dephosphorylation of S112 by phosphatases and occur immediately after dephosphorylation in a precisely coordinated manner. CNB-A/B, cyclic nucleotide binding domains.