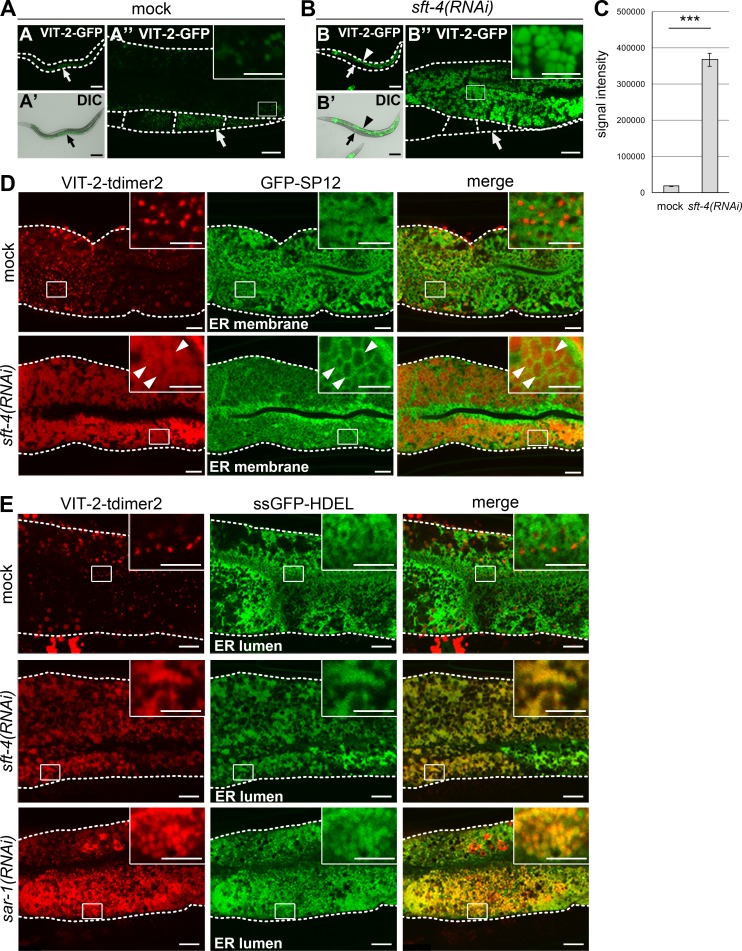
Figure 1.
SFT-4 is required for efficient ER export of VIT-2 from intestinal cells. (A–C) Loss of sft-4 caused high accumulation of VIT-2 in intestinal cells. (A–A″) In mock-treated animals, VIT-2–GFP is detected on certain small punctate structures in intestinal cells and is prominently accumulated in oocytes (arrows). (B–B″) VIT-2–GFP is highly accumulated in sft-4(RNAi) intestinal cells (arrowheads) but is almost undetectable in oocytes (arrows). (C) The amount of VIT-2–GFP in intestinal cells was significantly increased after knockdown of sft-4. Fluorescence signal intensities per unit area were measured and statistically analyzed using Student’s t test; ***, P < 0.001; error bars: SEM (n = 34 and 31 intestines from mock and sft-4(RNAi) animals, respectively). Dotted lines indicate the outlines of worm bodies (A and B) or intestines and oocytes (other panels). Regions surrounded by squares are enlarged (16×) in insets. Bars: (A, A′, B, and B′) 50 µm; (A″ and B″) 10 µm; (insets) 5 µm. (D and E) Subcellular localization of VIT-2 in mock and sft-4(RNAi) intestinal cells. Intestines of transgenic animals coexpressing VIT-2–tdimer2 and GFP-SP12 (D) or ssGFP-HDEL (E) are shown. L3 larvae were treated with RNAi for 2 d in the case of sar-1(RNAi). VIT-2–tdimer2 localizes to granular structures in the ER lumen labeled with ssGFP-HDEL in sft-4(RNAi) or sar-1(RNAi) intestinal cells. Dotted lines indicate the outlines of intestines. Regions surrounded by squares are enlarged (16×) in insets. Bars: 10 µm; (insets) 5 µm.