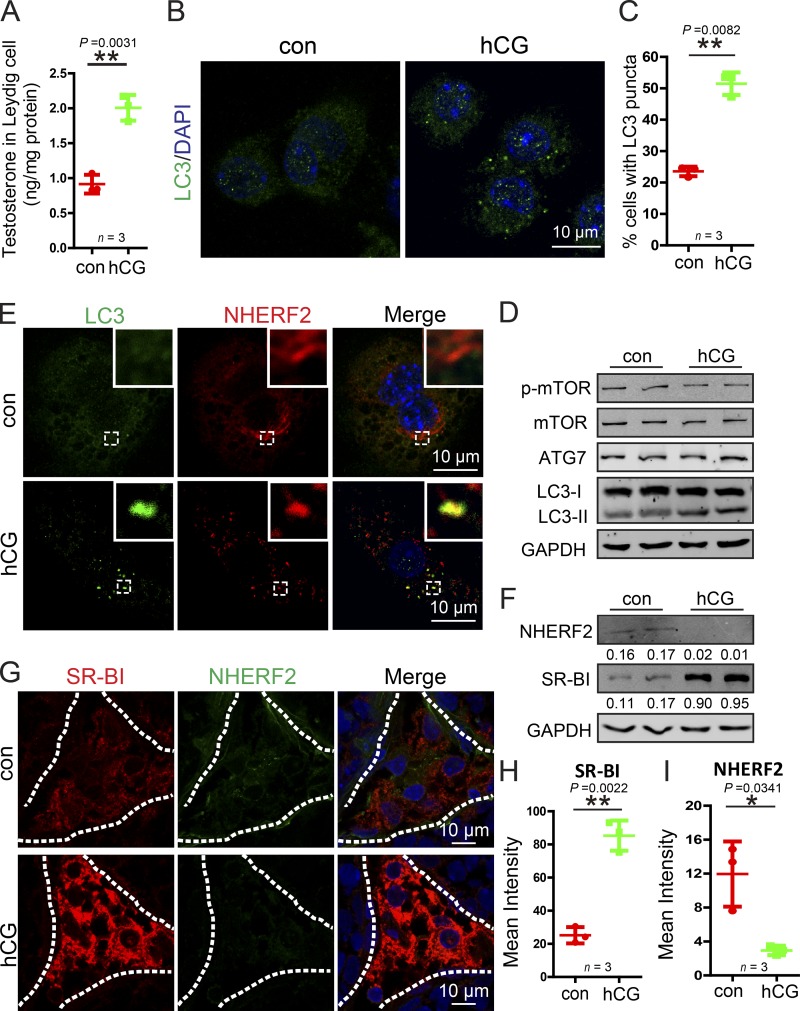
Figure 8.
hCG treatment enhances autophagy to promote NHERF2 clearance and testosterone synthesis in Leydig cells. (A) Testosterone production was significantly increased in hCG-treated Leydig cells. (B) The LC3 puncta was significantly increased after hCG treatment in Leydig cells. Immunofluorescence analysis of LC3 (green) was performed in hCG-treated and control Leydig cells. (C) Quantification of the percentages of cells with LC3 puncta in B. (D) The membrane-associated form LC3-II was accumulated in hCG-treated Leydig cells. The immunoblotting analysis of phosphor-mTOR, mTOR, ATG7, and LC3 was performed in hCG-treated and control Leydig cells. GAPDH served as a loading control. (E) NHERF2 partially colocalized with LC3 in hCG-treated Leydig cells. Immunofluorescence analysis of NHERF2 (red) and LC3 (green) were performed in hCG-treated and control Leydig cells. Insets are marked by boxes and are 2.3 µM wide. (F) NHERF2 was reduced, and SR-BI accumulated in hCG-treated Leydig cells. The immunoblotting analysis of NHERF2 was performed in hCG-treated and control Leydig cells. GAPDH served as a loading control. Quantifications of Western blots for NHERF2 and SR-BI relative to GAPDH is shown below the corresponding bands. (G) A negative correlation between SR-BI and NHERF2 in hCG-treated and untreated Leydig cells. Immunofluorescence analysis of SR-BI (red) and NHERF2 (green) were performed in hCG-treated and control mice. Nuclei were stained with DAPI (blue). (H and I) Quantification of the fluorescence intensity of SR-BI and NHERF2 in G. *, P < 0.05; **, P < 0.01.