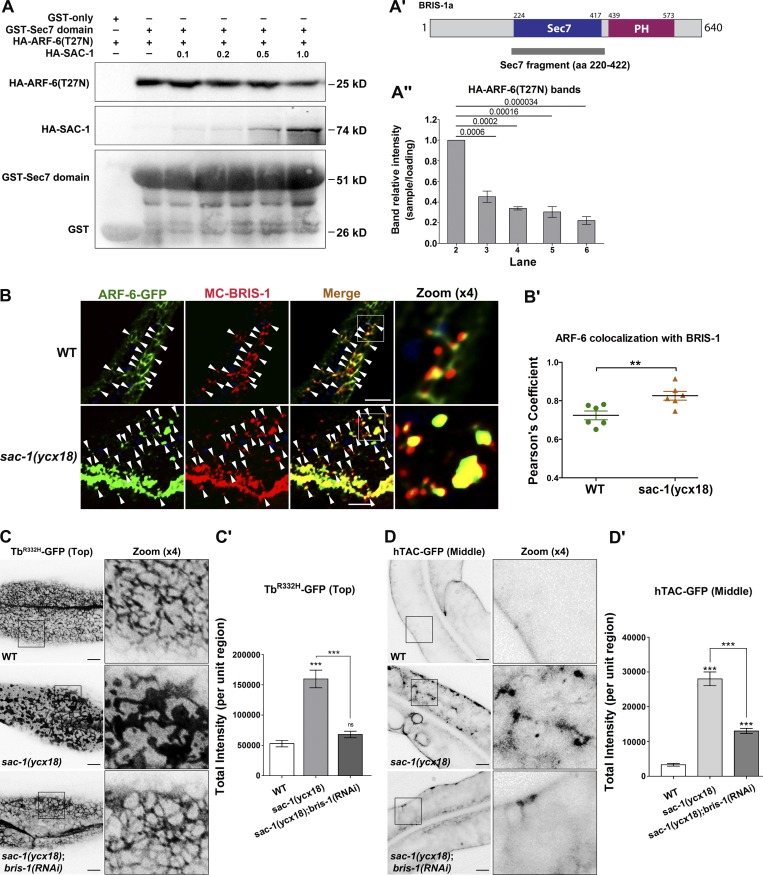
Figure 6.
SAC-1 competes with ARF-6(T27N) for binding to the Sec7 domain of BRIS-1. (A–A″) SAC-1 outcompeted ARF-6(T27N) for interaction with the Sec7 domain in a concentration-dependent manner, as determined by GST pull-down. Three independent experiments are represented in the histogram, error bars represent SEM (n = 3 each), and P values are indicated above. (B and B′) In wild-type animals, mCherry-BRIS-1 mainly labeled basolateral tubules and puncta and partially colocalized with ARF-6-GFP (Pearson’s coefficient, ∼72.4%). In sac-1(ycx18) mutants, mCherry-BRIS-1 and ARF-6-GFP were concentrated in the intracellular aggregates, and their colocalization level was substantially promoted (Pearson’s coefficient, ∼85.4%). Arrowheads indicate positive overlap. Pearson’s correlation coefficients for GFP and mCherry signals were calculated (n = 6 animals). Asterisks indicate significant differences in the one-tailed Student’s t test (**, P < 0.01). (C and C′) Compared with wild-type animals, the labeling intensity of TbR332H-GFP in the basolateral endosomes was grossly increased in sac-1(ycx18) mutants. Furthermore, RNAi-mediated knockdown of BRIS-1 significantly eased the intensity of TbR332H-GFP in sac-1 mutants. Error bars represent SEM (n = 18 each), and asterisks indicate significant differences in the one-tailed Student’s t test (***, P < 0.001). (D and D′) RNAi-mediated knockdown of BRIS-1 greatly reduced the accretion of hTAC-GFP in sac-1 mutants. Error bars represent SEM (n = 18 each), and asterisks indicate the significant differences in the one-tailed Student’s t test (***, P < 0.001). Bars, 10 µm.