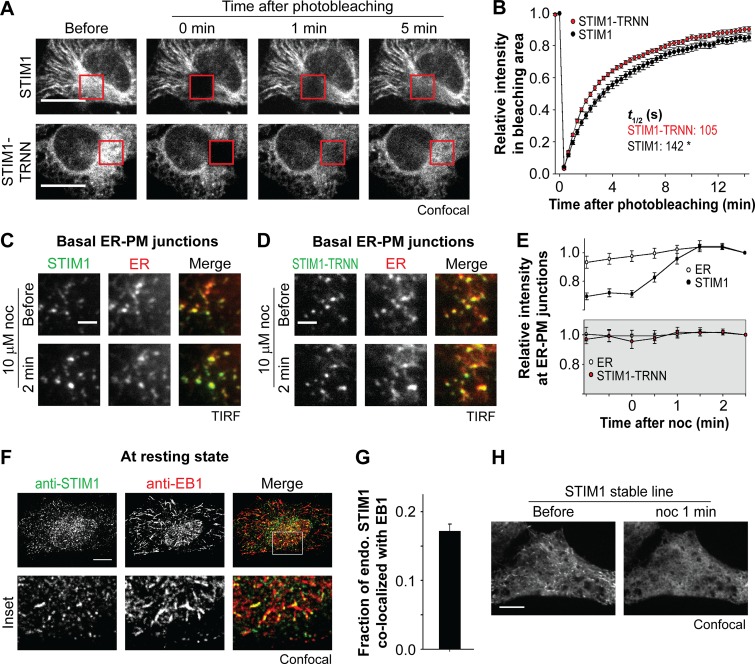
Figure 3.
EB1 binding constitutes a trapping mechanism limiting STIM1 localization at ER–PM junctions. (A) Fluorescence recovery of YFP-STIM1 and YFP-STIM1-TRNN after photobleaching (red square boxes) in HeLa cells, monitored by confocal microscopy. (B) Relative intensity of YFP-STIM1 and YFP-STIM1-TRNN in the bleached areas as described in A. 19–20 cells from three independent experiments. Mean times to the half recovery (t1/2) are indicated. *, P < 0.05. (C and D) Changes in intensity of YFP-STIM1 (C) and YFP-STIM1-TRNN (D) at ER–PM junctions after 10 µM nocodazole (noc) treatment, monitored by TIRF microscopy in HeLa cells cotransfected with mCherry-ER. (E) Relative changes in intensity of STIM1 subtypes and mCherry-ER at ER–PM junctions derived from relative single puncta intensity as described in C and D. Intensity at the end time point is defined as 1. 13–14 cells from three to four independent experiments. (F) Localization of endogenous STIM1 and EB1 at resting state, visualized by immunostaining using confocal microscopy. (G) Fraction of STIM1 overlapping with EB1 calculated from quantification of endogenous (endo.) STIM1 and EB1 colocalization by immunostaining as described in F. Means ± SEM are shown. 29 cells from two independent experiments. (H) Changes in localization of YFP-STIM1 after 10 µM nocodazole treatment, monitored by confocal microscopy in HeLa cells stably expressing YFP-STIM1 at a low level. Bars: (A, F, and H) 10 µm; (C and D) 2 µm.