Figure 3.
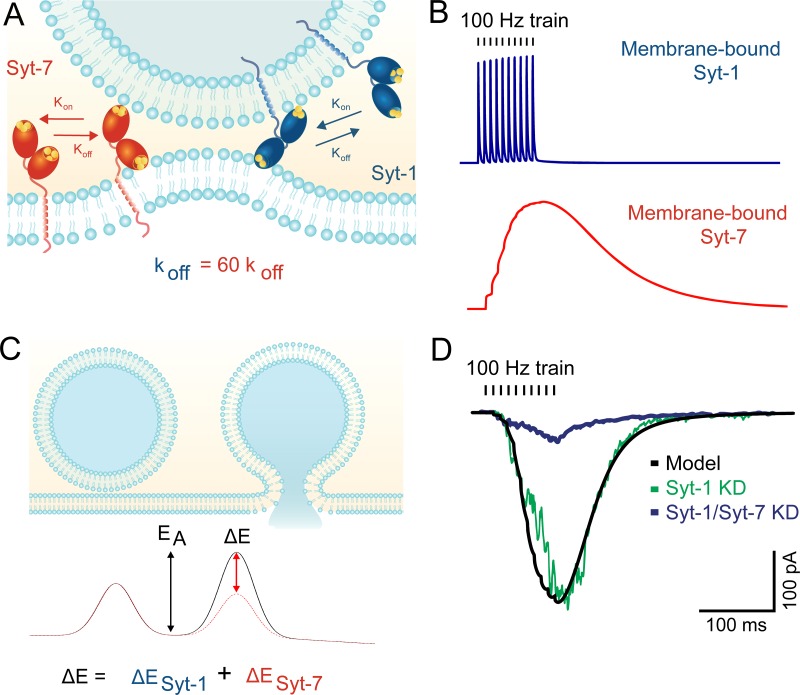
Membrane-binding kinetics of Syt-7 may explain its role in asynchronous release. (A) The C2 domains of Syt-1 and Syt-7 both bind membranes in a Ca2+-dependent manner. However, Syt-7 binds Ca2+ with much higher affinity and exhibits far slower unbinding kinetics after Ca2+ levels subside. (B) Modeled membrane binding of the two isoforms in response to a 100-Hz train of action potentials. Syt-1 rapidly releases from membranes in between action potentials, whereas Syt-7 binding continues to increase during the train. See Jackman and Regehr (2017) for modeling parameters. (C) For vesicles to fuse with the plasma membrane, they must overcome the energy barrier associated with merging the two lipid bilayers. Syt-1 (and likely Syt-7) lowers the barrier by bringing the two membranes in close apposition and inducing membrane curvature. (D) Modeling fusion rates without a contribution from Syt-1 (black line) predicts release rates that display similarities to synaptic responses recorded from Syt-1 KO neurons (adapted with permission from Bacaj et al., 2013). Syt-7 knockdown profoundly reduced release, indicating that the asynchronous release is driven mostly by Syt-7.