The F88L mutation in cardiac troponin T (TnTF88L) is associated with hypertrophic cardiomyopathy. Reda and Chandra reveal that it abolishes length-mediated increase in myofilament Ca2+ sensitivity and attenuates cooperative mechanisms governing length-dependent activation.
Abstract
Recent clinical studies have revealed a new hypertrophic cardiomyopathy–associated mutation (F87L) in the central region of human cardiac troponin T (TnT). However, despite its implication in several incidences of sudden cardiac death in young and old adults, whether F87L is associated with cardiac contractile dysfunction is unknown. Because the central region of TnT is important for modulating the muscle length–mediated recruitment of new force-bearing cross-bridges (XBs), we hypothesize that the F87L mutation causes molecular changes that are linked to the length-dependent activation of cardiac myofilaments. Length-dependent activation is important because it contributes significantly to the Frank–Starling mechanism, which enables the heart to vary stroke volume as a function of changes in venous return. We measured steady-state and dynamic contractile parameters in detergent-skinned guinea pig cardiac muscle fibers reconstituted with recombinant guinea pig wild-type TnT (TnTWT) or the guinea pig analogue (TnTF88L) of the human mutation at two different sarcomere lengths (SLs): short (1.9 µm) and long (2.3 µm). TnTF88L increases pCa50 (−log [Ca2+]free required for half-maximal activation) to a greater extent at short SL than at long SL; for example, pCa50 increases by 0.25 pCa units at short SL and 0.17 pCa units at long SL. The greater increase in pCa50 at short SL leads to the abolishment of the SL-dependent increase in myofilament Ca2+ sensitivity (ΔpCa50) in TnTF88L fibers, ΔpCa50 being 0.10 units in TnTWT fibers but only 0.02 units in TnTF88L fibers. Furthermore, at short SL, TnTF88L attenuates the negative impact of strained XBs on force-bearing XBs and augments the magnitude of muscle length–mediated recruitment of new force-bearing XBs. Our findings suggest that the TnTF88L-mediated effects on cardiac thin filaments may lead to a negative impact on the Frank–Starling mechanism.
Introduction
Hypertrophic cardiomyopathy (HCM) mutations in the central region (CR; residues 80–180) of cardiac troponin T (TnT) are not well tolerated, perhaps because of their negative impact on thin filament cooperativity and length-dependent activation of cardiac myofilaments. Previous work from our laboratory has demonstrated that the structural integrity of CR is important for thin filament cooperativity and length-dependent activation of cardiac myofilaments (Ford et al., 2012; Gollapudi and Chandra, 2016a,b). Recent studies have suggested that impairment of length-dependent activation may be one of the primary mechanisms by which HCM mutations lead to altered cardiac phenotype and deleterious complications associated with HCM (Sequeira et al., 2013). Clinical data have recently discovered a mutation in the CR of human TnT (F87L) that is associated with HCM and high incidences of sudden cardiac death in young and old adults (Gimeno et al., 2009). However, cardiac contractile dysfunction associated with the F87L mutation in human TnT is unknown.
Mutation-mediated impairment of length-dependent activation may have significant implications for intact heart function because length-dependent activation underlies the molecular basis for the Frank–Starling mechanism, which describes the ability of the heart to regulate cardiac output in response to beat-to-beat variations in end-diastolic volume (Allen and Kentish, 1985; Plotnick et al., 1986; Holubarsch et al., 1996; Konhilas et al., 2002a; Nowak et al., 2007; Abraham et al., 2016). Indeed, impairment of length-dependent activation has been observed in several cases of cardiomyopathies (van Dijk et al., 2012) and failing human hearts (Schwinger et al., 1994; Sequeira et al., 2013). At the myofilament level, cardiac muscle exhibits pronounced length-dependent activation, whereby an increase in sarcomere length (SL) augments myofilament Ca2+ sensitivity (Allen and Kentish, 1985; Wang and Fuchs, 1994; Konhilas et al., 2002a), leading to the recruitment of force-bearing cross-bridges (XBs) and an increase in myocardial force production. Such SL-mediated enhancement of myofilament Ca2+ sensitivity is regulated by cooperative mechanisms that feed back to activate cardiac thin filaments in response to an increase in SL (Allen and Kentish, 1985; Fitzsimons and Moss, 1998; Moss and Fitzsimons, 2002; Smith et al., 2009). In this context, interventions that increase myofilament Ca2+ sensitivity and/or alter XB-based cooperative mechanisms have been shown to alter SL-dependent function in cardiac muscle (Fitzsimons and Moss, 1998; Arteaga et al., 2000; Ford et al., 2012; Kobirumaki-Shimozawa et al., 2014). For example, a previous study has demonstrated that the R92L mutation in the CR of mouse TnT enhances myofilament Ca2+ sensitivity but abolishes the SL-mediated increase in myofilament Ca2+ sensitivity (Ford et al., 2012). Because thin filament cooperativity and muscle length (ML)-mediated XB recruitment dynamics are significantly modulated by the CR of TnT (Gollapudi et al., 2013), HCM-associated mutations in the CR are expected to have a negative impact on length-dependent activation.
To investigate the effect of F87L on contractile function and whether such effects varied in an SL-dependent manner, recombinant guinea pig wild-type TnT (TnTWT) and mutant TnT (TnTF88L) were generated and reconstituted into detergent-skinned papillary muscle fibers isolated from guinea pig left ventricles. Guinea pig cardiac tissue was preferred because guinea pigs, like humans (Narolska et al., 2005a,b), predominantly express the β-myosin heavy chain (β-MHC) isoform in the myocardium (van der Velden et al., 1998). MHC isoform becomes an important aspect to consider when assessing the effect of mutations in the CR because the effects of CR on contractile function are modulated differently by α- and β-MHC isoforms (Ford et al., 2012; Gollapudi and Chandra, 2016a,b). For example, R92L in TnT abolishes the length-dependent increase in myofilament Ca2+ sensitivity in β-MHC–expressing fibers, but not in α-MHC–expressing fibers, suggesting that the effect of TnT-mediated changes on length-dependent activation depends on the type of MHC isoform (Ford et al., 2012). In addition, muscle fibers expressing β-MHC have been shown to exhibit steeper SL-dependent changes in dynamic contractile parameters (Korte and McDonald, 2007; Ford et al., 2012). Steady-state and dynamic contractile parameters were measured in TnTWT and TnTF88L muscle fibers at two different SLs (1.9 µm and 2.3 µm). Our results demonstrated that TnTF88L abolished the SL-mediated increase in cardiac myofilament Ca2+ sensitivity. Moreover, the magnitude of ML-mediated recruitment of XBs (ER) and the negative impact of strained XBs on recruitment of force-bearing XBs (γ) were altered in an SL-dependent manner.
Materials and methods
Animal protocols
8–10-mo-old male Dunkin-Hartley guinea pigs (Cavia porcellus) acquired from Charles River were used in this study. All animals were housed in environmentally controlled rooms of an Association for Assessment and Accreditation of Laboratory Animal Care–accredited facility under 12-h light and dark cycles. All animals received proper care and treatment in accordance with the preapproved protocols by Washington State University Institutional Animal Care and Use Committee. The procedures for euthanizing guinea pigs conformed to the recommendations of the American Veterinary Medical Association, as outlined in the Guidelines for the Euthanasia of Animals.
Expression and purification of recombinant guinea pig cardiac troponin subunits
Recombinant c-myc–tagged guinea pig TnT (TnTWT and TnTF88L), guinea pig WT troponin I (TnI), and guinea pig WT troponin C (TnC) were generated and cloned into a pSBETa vector (GenScript USA). The inclusion of the c-myc tag has been previously shown to have no effect on contractile function (Tardiff et al., 1998; Montgomery et al., 2001; Chandra et al., 2005). Recombinant DNA was transformed and expressed in BL21*DE3 cells (Invitrogen). In brief, TnTWT and TnTF88L were purified by ion-exchange chromatography on a DEAE-Fast Sepharose column (GE Healthcare Biosciences). TnI was purified using a CM Sepharose ion-exchange column, and TnC was purified using a DE-52 column and phenyl Sepharose column. Details on protein purification can be found in the Supplemental materials and methods.
Reconstitution of recombinant troponin complexes in detergent-skinned guinea pig cardiac muscle fibers
Left ventricular guinea pig papillary muscle fibers were prepared and detergent skinned, as described in the Supplemental materials and methods. Reconstitution of recombinant troponin into cardiac muscle fibers was done as previously described (Chandra et al., 1999; Gollapudi et al., 2012; Mamidi et al., 2013). Recombinant TnTWT or TnTF88L (0.9 mg/ml, wt/vol) and TnI (1.0 mg/ml, wt/vol) were dissolved in an exchange buffer containing the following: 50 mM Tris-HCl, 6 M urea, and 1.0 mM KCl, pH 8.0, at 4°C (buffer 1). To remove high salt and urea, proteins were successively dialyzed against 50 mM Tris-HCl, 4 M urea, and 0.7 M KCl, pH 8.0, at 4°C (buffer 2); 50 mM Tris-HCl, 2 M urea, and 0.5 M KCl, pH 8.0, at 4°C (buffer 3); and 50 mM N,N-bis (2-hydroxyethyl)-2-aminoethanesulfonic acid (BES), 200 mM KCl, 10 mM 2,3-butanedione monoxime (BDM), 6.27 mM MgCl2, and 5 mM EGTA, pH 7.0, at 22°C (buffer 4). All buffers included a cocktail of protease inhibitors (0.2 mM PMSF, 2 mM benzamidine-HCl, 1 mM dithiothreitol (DTT), and 0.01% sodium azide). Any undissolved protein in the exchange buffer was removed by centrifugation at 3,000 rpm for 15 min. Detergent-skinned fiber bundles were treated with the exchange buffer containing TnTWT + TnI or TnTF88L + TnI for ∼3 h at room temperature (22°C) with gentle stirring. Muscle fiber bundles were then washed twice (10 min each) with buffer 4 to remove remnants of exchange buffer, followed by overnight incubation at 4°C in high-relaxing solution containing TnC (3.0 mg/ml, wt/vol).
Measurement of isometric steady-state tension and ATPase activity
Steady-state isometric tension was measured in muscle fibers at various pCa values (−log10 of [Ca2+]free), as previously described (de Tombe and Stienen, 1995; Stienen et al., 1995; Campbell et al., 2004; Gollapudi et al., 2015). The composition of pCa solutions can be found in the Supplemental materials and methods. In brief, muscle fibers were mounted between a motor arm (322C; Aurora Scientific) and a force transducer (AE 801; Sensor One Technologies). SL was set to 1.9 or 2.3 µm in high-relaxing solution using He-Ne laser diffraction technique (de Tombe and Stienen, 1995). Each muscle fiber was exposed to various pCa solutions ranging from 4.3 to 9.0 in a constantly stirred chamber maintained at a temperature of 20°C. Force responses were recorded on a computer at a sampling rate of 1 kHz. Isometric steady-state tension was calculated at each pCa and normalized to maximal tension (tension at pCa 4.3). Normalized steady-state tension was then plotted against respective pCa to construct the pCa–tension relationship. For each muscle fiber, the Hill equation was fitted to the normalized pCa–tension data to estimate two parameters, pCa50 and nH, indices of myofilament Ca2+ sensitivity and myofilament cooperativity, respectively.
ATPase activity was measured using an enzymatically coupled reaction that couples the breakdown and regeneration of ATP to the oxidation of NADH (de Tombe and Stienen, 1995; Stienen et al., 1995; Chandra et al., 2006). In brief, near-UV light was projected through the measuring chamber, and a beam splitter (50:50) was used to detect wavelengths of 340 nm (sensitive to changes in NADH) and 400 nm (insensitive to changes in NADH). Because the oxidation of NADH is enzymatically coupled to the breakdown and regeneration of ATP, changes in ATPase activity can be estimated from changes in NADH, which is measured by changes in UV absorbance at 340 nm. The UV absorbance signal of NADH was calibrated by multiple injections of 250 pmol ADP into the measuring chamber. Tension cost was derived from the ATPase–tension relationship, as previously described (de Tombe and Stienen, 1995; Chandra et al., 2015).
Measurement of muscle fiber mechanodynamics
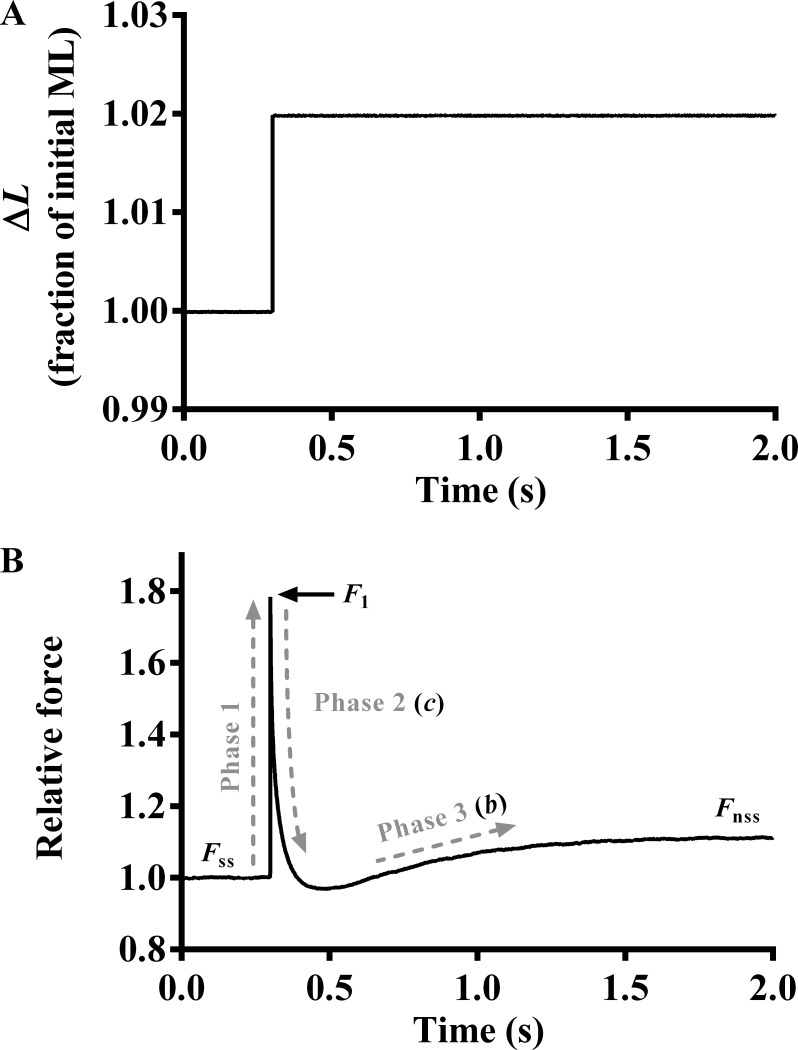
Upon attainment of maximal steady-state activation, the motor arm was commanded to elicit various amplitude stretch/release perturbations (±0.5, ±1.0, ±1.5, and ±2.0% of the initial ML) to the attached muscle fibers (Ford et al., 2010). Each length perturbation was maintained for 5 s, and the corresponding force responses were recorded to highlight three different phases. As described previously (Ford et al., 2010), a nonlinear recruitment-distortion (NLRD) model was fitted to the force response phases to estimate five model parameters: the magnitude of the instantaneous increase in force caused by a sudden increase in ML (ED); the rate by which force drops because of dissipation of strain within bound XBs (c); a nonlinear interaction term representing the negative effect of strained XBs on other force-bearing XBs (γ); the rate by which XBs are recruited into the force-bearing state at the new ML (b); and the magnitude of increase in steady-state force caused by recruitment of additional force-bearing XBs at the increased ML (ER). Fig. 1 depicts the length protocol of 2% sudden stretch (Fig. 1 A) and the corresponding force response (Fig. 1 B) from a muscle fiber. Details on the physiological significance of each model parameter can be found in the Supplemental materials and methods.
Figure 1.
Representative force response to a sudden 2% stretch. (A) A representative 2% sudden stretch in ML imposed on a TnTWT muscle fiber at maximal Ca2+ activation (pCa 4.3). (B) The corresponding force response normalized to the isometric steady-state force (Fss) before ML change. The NLRD model was fitted to the family of force responses to steplike changes in ML (±0.5, ±1.0, ±1.5, and ±2.0% of initial ML) to estimate various model parameters (Ford et al., 2010). The different phases (dashed lines) from which the respective parameters were estimated are highlighted. F1, the instantaneous increase in force caused by sudden increase in ML (phase 1); c, the rate by which force decays to a minimum point after a sudden stretch in ML (phase 2); γ, a nonlinear interaction term representing the negative impact of strained XBs on other force-bearing XBs; b, the rate of delayed force rise after an increase in ML (phase 3); Fnss, the new steady-state force corresponding to an increase in ML.
Rate constant of tension redevelopment
The rate constant of tension redevelopment (ktr) was estimated using a modified version of the large slack/restretch maneuver originally designed by Brenner and Eisenberg (1986) and is described in the Supplemental materials and methods.
Statistical analysis
Our experimental model investigated the effects of two factors, TnT (TnTWT and TnTF88L) and SL (1.9 and 2.3 µm). Thus, two-way ANOVA was constructed to analyze the effect of TnT on each contractile parameter at a given SL. A significant TnT–SL interaction effect suggested that the effect of TnTF88L on a given parameter was dissimilar at different SLs. When the interaction was not significant, we assessed the main effect of TnT at different SLs. To determine the source of significant interaction or main effect, post hoc t tests using Fisher’s least significant difference (LSD) method were analyzed. The criterion for statistical significance was set to P < 0.05. Data are presented as mean ± SEM.
Online supplemental material
Details regarding expression and purification of recombinant guinea pig cardiac troponin subunits, preparation of guinea pig cardiac muscle fibers, Western blot analysis of reconstituted muscle fiber samples, composition of pCa solutions, NLRD model parameters, and measurement of ktr can be found in the Supplemental materials and methods. Western blot analysis of reconstituted muscle fibers (Fig. S1) and contractile dynamic data regarding the effect of TnTF88L at submaximal activation (Table S1) are also included.
Results
Western blot analysis of recombinant TnT incorporation in guinea pig cardiac muscle fibers
To assess the level of recombinant TnT incorporation, reconstituted TnTWT or TnTF88L muscle fibers were solubilized in 2.5% SDS solution and run on 8% SDS gel to separate endogenous and recombinant (c-myc–tagged) TnT, as described in Supplemental materials and methods. The inclusion of the c-myc tag at the N terminus allowed us to separate recombinant TnT (TnTWT or TnTF88L) from the endogenous TnT via different migration on SDS gel. We and others have previously shown that the c-myc epitope at the N terminus of TnT does not affect cardiac function (Tardiff et al., 1998; Montgomery et al., 2001; Chandra et al., 2005). To quantify the level of incorporation of recombinant TnT (i.e., the amount of total endogenous TnT that was replaced by recombinant TnT) in reconstituted muscle fibers, we used Western blot analysis. Densitometric analysis of the band profiles from the Western blot revealed that the incorporation level of c-myc–tagged TnTWT was 77 ± 6%, and that of c-myc–tagged TnTF88L was 72 ± 7% (Fig. S1). Values reported as mean ± SD; n = 3.
Effect of TnTF88L on Ca2+-activated tension and ED at short and long SLs
To determine whether TnTF88L altered maximal tension and whether this effect varied in an SL-dependent manner, we assessed steady-state tension at maximal Ca2+ activation at both short and long SLs. Two-way ANOVA did not show a significant TnT–SL interaction effect (P = 0.28) or a significant TnT main effect (P = 0.66) on maximal tension (Table 1). To corroborate our findings on maximal tension, we assessed ED. Previous studies have shown that ED is strongly correlated to maximal tension and is therefore an index of the number of strongly bound XBs (Campbell et al., 2004; Ford et al., 2010). Similar to our findings on maximal tension, two-way ANOVA did not show a significant TnT–SL interaction effect (P = 0.22) or a significant TnT main effect (P = 0.74) on ED. At short SL, ED was 639 mN/mm3 for TnTWT fibers and 690 mN/mm3 for TnTF88L fibers. At long SL, ED was 897 mN/mm3 for TnTWT fibers and 867 mN/mm3 for TnTF88L fibers. Observed changes in maximal tension and ED suggest that TnTF88L does not alter maximal activation.
Table 1. Effect of TnTF88L on various contractile parameters at short and long SLs.
| Parameter | 1.9 µm | 2.3 µm | ||
|---|---|---|---|---|
| TnTWT | TnTF88L | TnTWT | TnTF88L | |
| Maximal tension (mN ⋅ mm−2) | 29.72 ± 1.25 | 31.63 ± 1.52a | 49.07 ± 1.24 | 48.25 ± 0.93a |
| Tension cost (pmol ⋅ mN−1 ⋅ mm−1 ⋅ s−1) | 2.56 ± 0.17 | 2.34 ± 0.11a | 1.26 ± 0.06 | 1.32 ± 0.07a |
| c (s−1) | 12.17 ± 0.42 | 10.98 ± 0.89a | 7.95 ± 0.13 | 8.10 ± 0.38a |
| b (s−1) | 4.32 ± 0.09 | 4.19 ± 0.09a | 4.33 ± 0.11 | 4.37 ± 0.09a |
| ktr (s−1) | 1.99 ± 0.05 | 1.83 ± 0.11a | 1.61 ± 0.10 | 1.57 ± 0.05a |
Maximal tension was measured by exposing muscle fibers to saturating Ca2+ concentration (pCa 4.3) in a constantly stirred chamber. Tension cost was derived from the ATPase/tension relationship, as previously described (de Tombe and Stienen, 1995; Chandra et al., 2015). Parameters c and b were estimated by fitting the NLRD model to the force response phases to various amplitude stretch/release perturbations (Fig. 1; Ford et al., 2010). c represents the rate of force decay to a minimum force point following a sudden stretch in ML, and b represents the rate at which force rises at the new ML. ktr was estimated by fitting a monoexponential function to the rising phase of the force response to a large slack-restretch length maneuver and represents the rate of tension redevelopment (Brenner and Eisenberg, 1986). Estimates from several muscle fibers per group were averaged and presented as mean ± SEM. Statistical differences were analyzed using two-way ANOVA and subsequent post hoc multiple pairwise comparisons (Fisher’s LSD method). The number of fibers measured (from three hearts) for all groups was 10.
Not significantly different from TnTWT fibers.
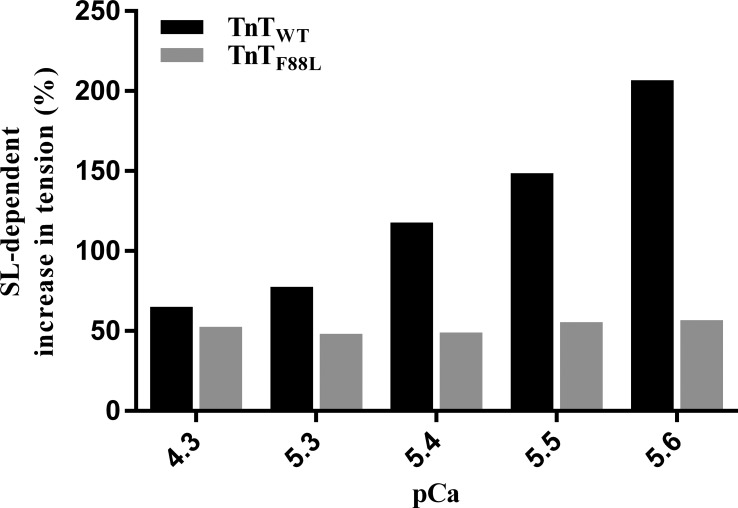
Although TnTF88L did not alter maximal activation, we observed a substantial effect on tension at submaximal activations (pCa 5.6). At short SL, tension at pCa 5.6 was 4.59 mN/mm2 for TnTWT fibers and 17.33 mN/mm2 for TnTF88L fibers. At long SL, tension at pCa 5.6 was 14.02 mN/mm2 for TnTWT fibers and 27.16 mN/mm2 for TnTF88L fibers. Two-way ANOVA of tension at pCa 5.6 did not show a significant interaction effect (P = 0.85), but the main effect of TnT was significant (P < 0.001). TnTF88L increased tension at pCa 5.6 to a greater extent at short SL (278%, P < 0.001) than at long SL (93%; P < 0.001). Because of this greater increase in tension at short SL, the SL-dependent increase in tension at pCa 5.6 was ∼3.6-fold less in TnTF88L fibers compared with TnTWT fibers. The SL-mediated increase in tension at pCa 5.6 was 207% in TnTWT fibers (P < 0.001), whereas it was only 57% in TnTF88L fibers (P < 0.001). Our data on submaximal tension demonstrates that the SL-mediated increase in force is significantly attenuated by TnTF88L, suggesting that TnTF88L may blunt ML-mediated increase in force production in heart muscle, which normally operates under submaximal activating conditions.
Effect of TnTF88L on pCa50 and nH at short and long SLs
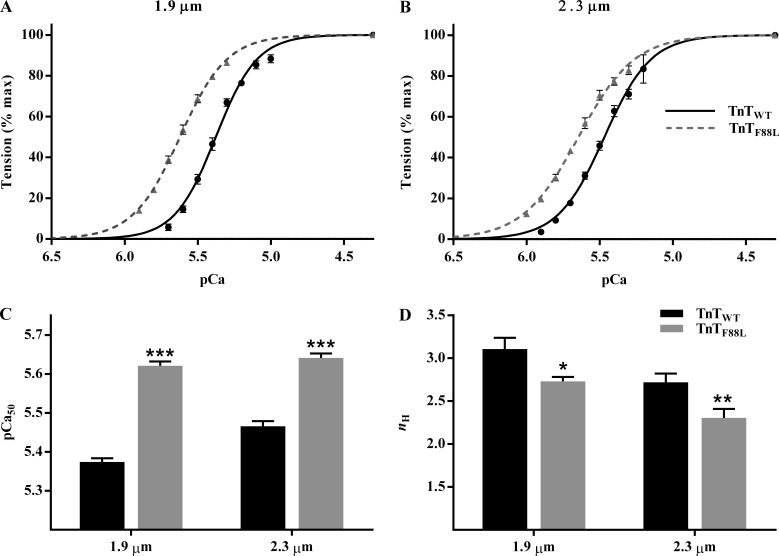
A comparison of pCa–tension relationships shows a leftward shift in the pCa–tension relationship of TnTF88L muscle fibers at both short SL (Fig. 2 A) and long SL (Fig. 2 B). A cursory look indicates that the magnitude of leftward shift in pCa–tension relationship is greater at short than at long SL, suggesting that TnTF88L increases myofilament Ca2+ sensitivity to a greater extent at short SL than at long SL. To quantify the magnitude of TnTF88L-mediated effect on pCa–tension relationship, we used Hill model–derived parameters, pCa50 and nH. Two-way ANOVA revealed a significant TnT–SL interaction effect (P = 0.003) on pCa50, suggesting that the effect of TnTF88L on pCa50 is dissimilar at different SLs. Post hoc t tests indicated that TnTF88L significantly increased pCa50 to a greater extent at short SL (0.25 units; P < 0.001; Fig. 2 C) than at long SL (0.17 units; P < 0.001; Fig. 2 C). This differential effect on pCa50 impacted the SL dependence of pCa50 in TnTF88L muscle fibers, such that pCa50 was not different between short and long SL in TnTF88L muscle fibers. Although increasing SL from 1.9 to 2.3 µm significantly increased pCa50 by 0.10 units in TnTWT fibers (P < 0.001; Fig. 2 C), it did not significantly increase pCa50 in TnTF88L fibers (P = 0.22; Fig. 2 C), indicating that TnTF88L abolished the length-mediated increase in Ca2+ sensitivity, a major mechanism underlying cardiac length-dependent activation. With regard to nH, two-way ANOVA did not reveal a significant TnT–SL interaction effect on nH (P = 0.84); however, the main effect of TnT was significant (P < 0.001). Post hoc t tests revealed that TnTF88L attenuated nH at both short SL (12%; P = 0.011; Fig. 2 D) and long SL (15%; P = 0.005; Fig. 2 D), suggesting an impact on mechanisms governing myofilament cooperativity. This impact on myofilament cooperativity and abolishment of length-mediated increase in Ca2+ sensitivity blunted the SL-mediated increase in tension at all Ca2+ concentrations tested (Fig. 3).
Figure 2.
Effect of TnTF88L on pCa–tension relationship at short and long SLs. (A and B) A comparison of pCa–tension relationship between TnTWT and TnTF88L muscle fibers at short SL (A) and long SL (B). Steady-state tensions at various pCa values were normalized to the corresponding value at pCa 4.3 and plotted against pCa to derive pCa–tension data. Normalized pCa–tension data measured from several muscle fibers per group were averaged and presented as mean ± SEM. Error bars are smaller than symbols in some cases. (C and D) Bar graphs showing the effect of TnTF88L on myofilament Ca2+ sensitivity (C; pCa50) and cooperativity (D; nH) at short and long SLs. Normalized pCa–tension data from each muscle fiber was individually fitted to Hill’s model to derive pCa50 and nH. Estimates from several fibers per group were averaged and presented as mean ± SEM. Two-way ANOVA revealed a significant TnT–SL interaction effect (P = 0.003) on pCa50. Two-way ANOVA did not show a significant TnT–SL interaction effect on nH (P = 0.84); however, the main effect of TnT was significant (P < 0.001). Post hoc multiple pairwise comparisons (Fisher’s LSD) were used to determine significant differences between groups. Asterisks indicate significant difference from TnTWT at a given SL (*, P < 0.05; **, P < 0.01; ***, P < 0.001). The numbers of fibers measured (from three hearts) for TnTWT and TnTF88L at short SL were 10 and 10, and those at long SL were 9 and 10, respectively.
Figure 3.
Effect of TnTF88L on SL-dependent increase in tension. Bar graph showing the percent increase in tension in response to an increase in SL from 1.9 to 2.3 µm in TnTWT (black) and TnTF88L (gray) fibers at various pCa values. For each pCa, steady-state tension values from muscle fibers per group were averaged, and percent difference between short and long SLs was calculated for TnTWT and TnTF88L groups. The minimum number of fibers measured (from three hearts) was nine per group.
Effect of TnTF88L on the rate of XB detachment at short and long SLs
Previous studies have shown that mutations in TnT may alter the rate of XB detachment, g (Chandra et al., 2015; Gollapudi and Chandra, 2016a; Reda et al., 2016). Changes in SL have also been shown to impact g (Chandra et al., 2006; Stelzer and Moss, 2006; Ford and Chandra, 2013), such that an increase in SL decreases g and vice versa. To determine whether TnTF88L altered g, and whether such effect varied in an SL-dependent manner, we assessed estimates of c and tension cost. Previous studies have demonstrated a strong correlation between c and tension cost (Campbell et al., 2004; Gollapudi and Chandra, 2016b), and both provide an appropriate measure of XB detachment rate, g. c represents the rate of XB distortion dynamics after a sudden stretch in ML and is estimated by fitting the NLRD model to the family of force responses to various amplitude length perturbations. Two-way ANOVA of c did not show a significant TnT–SL interaction effect (P = 0.20) or a significant main effect of TnT (P = 0.32; Table 1). Our observations on c were corroborated by our estimates of tension cost. Two-way ANOVA of tension cost did not reveal a significant TnT–SL interaction effect (P = 0.13) or a significant main effect of TnT (P = 0.65; Table 1). Collectively, findings on c and tension cost strongly suggest that TnTF88L does not alter g.
Effect of TnTF88L on XB turnover rate at short and long SLs
To determine whether TnTF88L altered the rate of XB turnover, and whether such effect varied in an SL-dependent manner, we assessed changes in two independent contractile parameters, b and ktr. b represents the rate constant of delayed force rise after an increase in ML and is estimated by fitting the NLRD model to a family of force responses to various amplitude step-like ML perturbations (Ford et al., 2010); ktr represents the rate of force redevelopment after a large release–restretch length maneuver (Brenner and Eisenberg, 1986). Previous studies have shown that b and ktr are reliable measures of XB turnover rate (Campbell et al., 2004). Two-way ANOVA of b did not show a significant TnT–SL interaction effect (P = 0.37) or a significant main effect of TnT (P = 0.65; Table 1). Two-way ANOVA of ktr did not show a significant TnT–SL interaction effect (P = 0.49) or a significant main effect of TnT (P = 0.24; Table 1). Collectively, our observations suggest that TnTF88L does not alter XB turnover rate.
Effect of TnTF88L on γ at short and long SLs
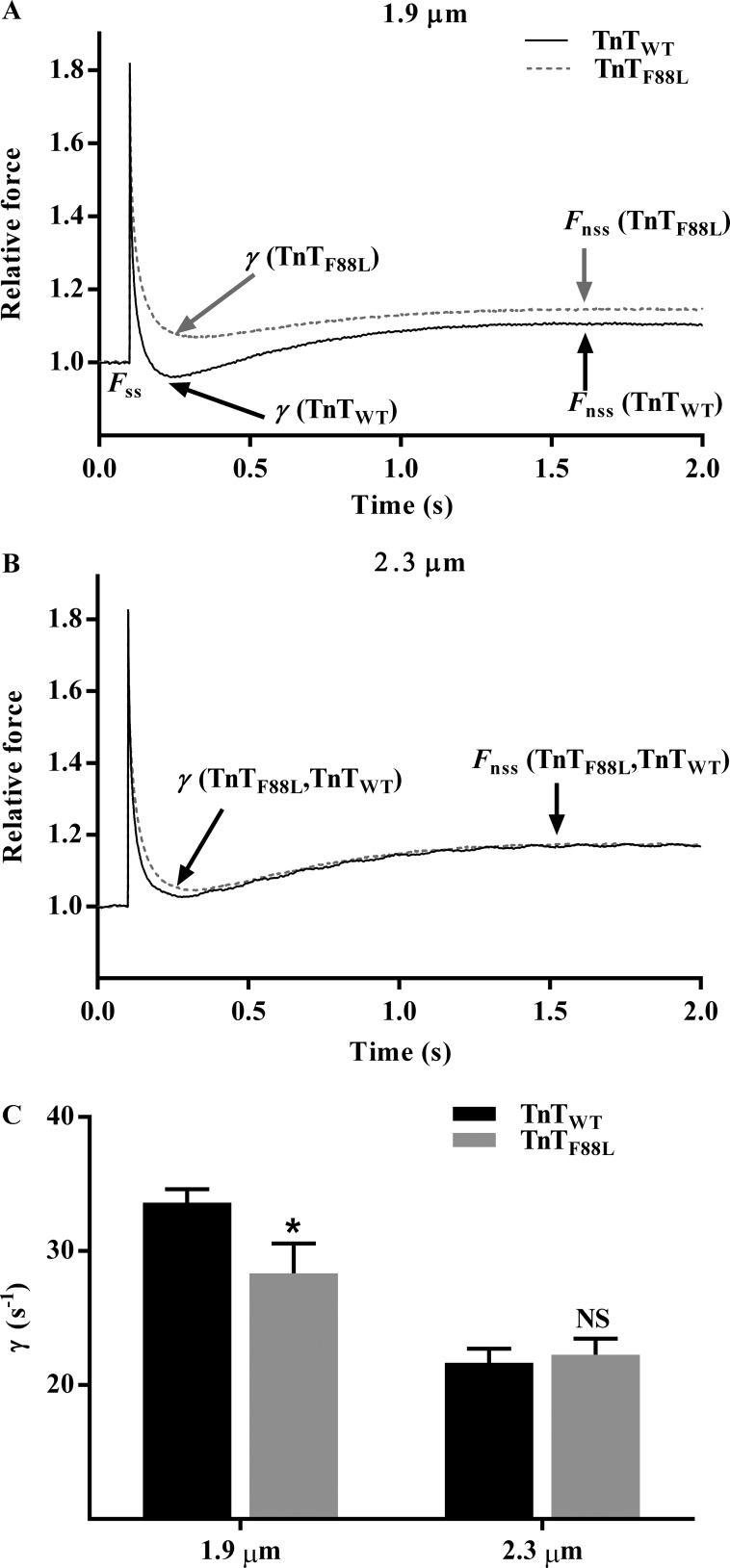
In our NLRD model, parameter γ represents the nonlinear interaction term, which is formulated as an effect by which the distortion of bound XBs influences the recruitment of other XBs. γ is estimated by fitting the NLRD model to a family of force responses to various amplitude length perturbations (Ford et al., 2010). From a physiological standpoint, γ is an indicator of the negative effect that strained XBs have on other force-bearing XBs. For example, when the negative impact of strained XBs on the state of other force-bearing XBs is less pronounced, the magnitude of force decline brought about by detachment of strained XBs is lesser (less prominent nadir), and thus γ is lower. Because such XB-based interactions are mediated by cooperative interactions within the thick and thin filaments, γ is thought to be influenced by allosteric/cooperative mechanisms in the myofilaments. A comparison of force responses to 2% sudden stretch shows that TnTF88L induces a less pronounced force decline at short SL but has no effect at long SL (Fig. 4, A and B). Two-way ANOVA of γ showed a marginally significant TnT–SL interaction effect on γ (P = 0.051), suggesting that TnTF88L altered γ differently at short and long SLs. Post hoc analysis showed that TnTF88L significantly decreased γ at short SL (16%; P = 0.015; Fig. 4 C); however, TnTF88L had no effect at long SL (P = 0.77; Fig. 4 C) when compared with TnTWT. Differential effects on γ suggest that the TnTF88L-mediated impact on thin filament–based allosteric/cooperative processes—which mediate XB-based cooperativity—is different at short and long SLs. Such differential effects attenuate the SL dependence of γ in TnTF88L fibers; this is demonstrated by the observation that when SL was increased from 1.9 to 2.3 µm, γ decreased by 36% (P < 0.001; Fig. 4 C) in TnTWT fibers but decreased by only 21% (P = 0.006; Fig. 4 C) in TnTF88L fibers, suggesting that SL-dependent effects on cooperative mechanisms that govern γ are attenuated by TnTF88L. We also assessed γ at comparable levels of force generation in TnTWT and TnTF88L fibers during submaximal activations. Our data at submaximal activations corroborate our findings at maximal activation; TnTF88L significantly decreased γ only at short SL (Table S1).
Figure 4.
Effect of TnTF88L on γ at short and long SLs. (A and B) Effect of TnTF88L on the force response to a 2% sudden stretch imposed on a representative muscle fiber at short SL (A) and long SL (B). Force data were normalized to the isometric steady-state value before ML perturbation, and length data were normalized to the initial ML. Fss represents the isometric steady-state value before ML perturbation, and Fnss represents the new steady-state force corresponding to an increase in ML. Parameter γ represents the negative impact of strained XBs on the recruitment of other force-bearing XBs and is estimated by fitting the NLRD model to the force response phases to various amplitude ML perturbations (Fig. 1; Ford et al., 2010). When the negative effect of strained XBs on the state of other force-bearing XBs is less pronounced, the magnitude of force decline is lesser; that is, the nadir is less pronounced and γ is attenuated. (C) Bar graph showing the effect of TnTF88L on γ at short and long SLs. Estimates from several muscle fibers per group were averaged and presented as mean ± SEM. Two-way ANOVA revealed a marginally significant TnT–SL interaction effect (P = 0.051) on γ. Post hoc multiple pairwise comparisons (Fisher’s LSD) were used to determine significant differences between groups. Asterisks indicate significant difference from TnTWT at a given SL (*, P < 0.05). The number of fibers measured (from three hearts) for all groups was 10.
Effect of TnTF88L on the magnitude of ML-mediated XB recruitment at short and long SLs
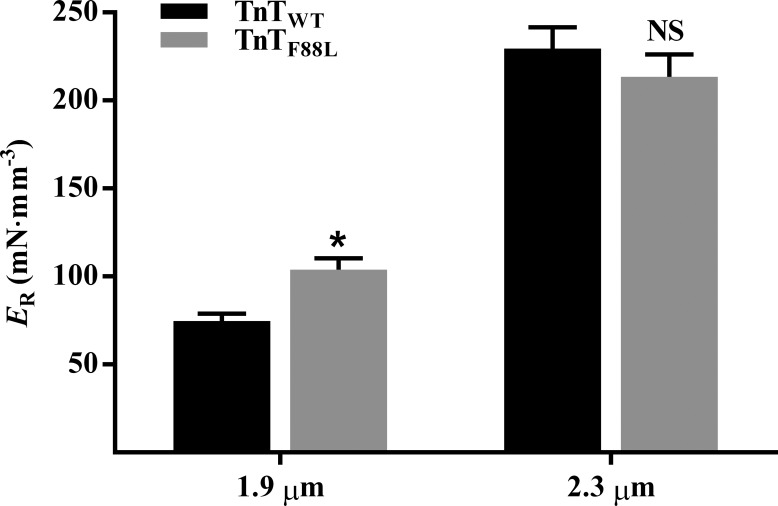
To determine the TnTF88L-mediated effect on the magnitude of ML-mediated XB recruitment, we assessed estimates of ER at maximal Ca2+ activation (pCa 4.3). ER represents the magnitude of delayed force rise in response to an increase in ML, an effect that is mediated by XB-based cooperative mechanisms (Campbell et al., 2004; Campbell and Chandra, 2006; Stelzer et al., 2006). ER is estimated by fitting the NLRD model to a family of force responses to various amplitude length perturbations (Ford et al., 2010). Two-way ANOVA revealed a significant TnT–SL interaction effect on ER (P = 0.031), suggesting that the effect of TnTF88L on ER was different at short and long SLs. Post hoc analysis confirmed that TnTF88L increased ER by 39% at short SL (P = 0.043; Fig. 5) but had no effect on ER at long SL (P = 0.25; Fig. 5), indicating that cooperative mechanisms governing ER were augmented at short SL. This dissimilar effect on ER at short and long SLs impacted the SL-mediated increase in ER; for example, post hoc analysis showed that increasing SL increased ER by 207% (P < 0.001; Fig. 5) in TnTWT fibers, but by only 105% (P < 0.001; Fig. 5) in TnTF88L fibers. Such an observation suggests that TnTF88L attenuates SL-dependent effects on cooperative mechanisms underlying ER. Here again, our data at submaximal activations corroborate our findings at maximal activation; TnTF88L significantly increased ER only at short SL (Table S1).
Figure 5.
Effect of TnTF88L on ER at short and long SLs. ER corresponds to the ML-mediated increase in steady-state force and represents the magnitude of ML-mediated XB recruitment. ER is estimated as the slope of the linear relationship between (Fnss−Fss) and ML changes, ΔL (see Figs. 1 and 4, A and B). Estimates from several muscle fibers per group were averaged and presented as mean ± SEM. Two-way ANOVA revealed a significant TnT–SL interaction effect (P = 0.031) on ER. Post hoc multiple pairwise comparisons (Fisher’s LSD) were used to determine significant differences between groups. Asterisks indicate significant difference from TnTWT at a given SL (*, P < 0.05). The numbers of fibers measured (from three hearts) for TnTWT and TnTF88L at short SL were 9 and 10, and those at long SL were 10 and 10, respectively.
Discussion
Data presented in this study provide novel insight regarding the effect of HCM-linked TnTF88L on contractile function and myofilament length-dependent activation. The major finding in our study is that the effect of TnTF88L mutation varies in an SL-dependent manner. Notably, TnTF88L increases pCa50 to a greater extent at short SL than at long SL, such that pCa50 is not different between short and long SLs in TnTF88L muscle fibers. That is, an increase in SL from 1.9 to 2.3 µm does not bring about the increase in pCa50 that is typically associated with length-dependent activation of cardiac myofilaments, suggesting an impact on mechanisms governing cardiac length-dependent activation. Given that length-dependent activation is a significant contributor to the Frank–Starling mechanism and that attenuated Frank–Starling mechanism is a hallmark of failing human hearts (Schwinger et al., 1994; van Dijk et al., 2012; Sequeira et al., 2013), our findings have important implications regarding cardiac dysfunction in patients harboring F87L.
Our observation that TnTF88L increases myofilament Ca2+ sensitivity to a greater extent at short SL than at long SL raises two important questions: (1) how does TnTF88L augment thin filament Ca2+ sensitivity, and (2) why is such an effect greater at short SL than at long SL? To address these questions, we must first consider mechanisms by which mutations in the CR of TnT may augment intrinsic thin filament responsiveness to Ca2+. TnTF88L is located in the CR, a region of TnT involved in allosteric/cooperative interactions that regulate Ca2+-mediated activation of thin filaments. Therefore, structural perturbations caused by mutations in the CR may affect processes regulating thin filament activation. In this regard, several lines of evidence show that mutations in the CR alter Ca2+ sensitivity of Tn by modifying allosteric interactions within the thin filament (Liu et al., 2012; Sommese et al., 2013; Williams et al., 2016); for example, I79N and E163K mutations in TnT increase Ca2+ affinity for Tn in reconstituted thin filament preparations containing actin:Tm:Tn (Sommese et al., 2013). However, I79N and E163K do not alter Ca2+ affinity for Tn in in vitro preparations containing TnT + TnI + TnC, suggesting two important points: (1) mutation-mediated effect on thin filament Ca2+ sensitivity is mediated by cooperative interactions within the thin filament, and (2) mechanisms other than a direct effect of the mutant on Tn Ca2+ sensitivity may be involved in altering thin filament Ca2+ sensitivity. Further support for this notion is provided by the observation that, although both R92L and R92W mutations in CR increase myofilament Ca2+ sensitivity in muscle fibers (Ford et al., 2012), the rate of Ca2+ dissociation from the isolated TnT + TnI + TnC complex is decreased by R92L but increased by R92W (Williams et al., 2016), suggesting that proper manifestation of the effect of CR mutations on myofilament Ca2+ sensitivity requires participation of allosteric/cooperative processes within the thin filament.
Structural changes in the CR of TnT may alter thin filament Ca2+ sensitivity by altering cooperative processes that affect the equilibrium between off/on states of regulatory unit (RU; Tn-Tm) located on actin filaments. CR not only promotes the binding of Tm to actin, but also aids in the assembly of Tm on the actin filament by promoting head-to-tail interactions between two contiguous Tms (Jackson et al., 1975; Heeley et al., 1987; Lehrer and Geeves, 1998; Palm et al., 2001). Therefore, CR plays a key role in mediating cooperative interactions between two neighboring RUs, which defines RU–RU cooperativity (Razumova et al., 2000). Thus, it is not surprising that mutations in CR modify RU–RU cooperativity by altering the coupling between CR and Tm (Palm et al., 2001; Hinkle and Tobacman, 2003). Because RU–RU cooperativity has the greatest effect on nH (Razumova et al., 2000), significant attenuation of nH is suggestive of a decrease in RU–RU cooperativity, which is expected to increase myofilament Ca2+ sensitivity (Razumova et al., 2000). To elaborate, at lower levels of Ca2+, when the majority of RUs are in the off state, cooperativity between two neighboring RUs tends to stabilize RUs in the off state. A consequence of this effect is that it requires more Ca2+ to activate RUs, which results in decreased Ca2+ sensitivity. When cooperative stabilization between neighboring RUs is decreased by TnTF88L, it decreases the threshold for Ca2+ to activate RUs, leading to increased Ca2+ sensitivity of thin filaments. Such attenuation of RU–RU cooperativity may result from the weakening of TnT–Tm interactions (Gangadharan et al., 2017) and/or an effect on Tm–Tm overlap junction; in theory, this would lower the effective stiffness of the Tm chain and decrease cooperative communication between RUs. This line of reasoning is consistent with previous findings that have suggested that one primary mechanism by which HCM-linked thin filament mutations enhance myofilament Ca2+ sensitivity is through attenuation of RU–RU cooperativity (Palm et al., 2001; Hinkle and Tobacman, 2003). Thus, a substantial increase in Ca2+ sensitivity in TnTF88L fibers may be attributed to alterations in thin filament allosteric/cooperative mechanisms brought about by structural changes in RU.
Although the above explanation provides a molecular basis for the TnTF88L-mediated effect on thin filament Ca2+ sensitivity, it does not explain the differential effects observed at short and long SLs. Differential effects on tension at short and long SLs during submaximal activation may help explain the lack of SL-dependent effect on myofilament Ca2+ sensitivity in TnTF88L fibers. Notably, at pCa 5.6, TnTF88L augments tension by 278% at short SL and by 93% at long SL, demonstrating that the ability of TnTF88L to increase the number of force-bearing XBs at submaximal activations is substantially greater at short SL. A consequence of such an increase in the number of strongly bound XBs at submaximal activation is that TnTF88L enhances XB-based cooperative mechanisms (XB–XB and XB–RU) more at short SL. Therefore, we posit that TnTF88L enhances XB-based cooperative feedback effect on thin filament to a greater degree at short SL. Because an increase in XB-based cooperativity is important for the SL-dependent increase in myofilament Ca2+sensitivity (Allen and Kentish, 1985; Wang and Fuchs, 1994; Fitzsimons and Moss, 1998; Konhilas et al., 2002b; Smith et al., 2009), the lack of an increase in Ca2+ sensitivity between short and long SL suggests that XB-based cooperative mechanisms are saturated at short SL in TnTF88L fibers. A consequence of proper manifestation of XB-based cooperativity is that the SL-mediated increase in tension (that is, an increase in tension associated with an increase in SL from 1.9 to 2.3 µm) is more pronounced at lower Ca2+ concentrations (Campbell, 1997; Razumova et al., 2000). Indeed, the SL-dependent increase in tension in TnTWT fibers is more pronounced at lower Ca2+ activations because the SL-dependent increase in XB-based cooperativity is normal in TnTWT fibers (Fig. 3). However, this SL-mediated increase in tension is absent in TnTF88L fibers at all Ca2+concentrations tested, suggesting that XB-based cooperative mechanisms are saturated at short SL in TnTF88L fibers. To clarify, the TnTF88L-mediated increase in XB-based cooperativity, combined with an increase in RU activation, causes a depletion in the number of RUs and XBs available for cooperative recruitment. Consequently, it limits the number of RUs and XBs that can be effectively engaged to enhance XB-based cooperative mechanisms as SL increases.
Additional evidence for increased XB-based cooperativity at short SL and disruption of SL-mediated effects on XB-based cooperativity may be gleaned from our observations on two other contractile dynamic parameters, γ and ER. Parameter γ represents thin filament–based regulatory mechanisms by which strained XBs negatively impact other force-bearing XBs (Ford et al., 2010). A significant decrease in γ at short SL in TnTF88L fibers suggests that the negative effect of strained XBs on force-bearing XBs is attenuated, a likely consequence of greater expression of XB-based cooperativity. We posit that increased XB-based cooperativity counteracts the negative impact of strained XBs on force-bearing XBs, leading to a decrease in γ at short SL. The augmenting effect of TnTF88L on XB-based cooperativity at short SL is also supported by the magnitude of ML-mediated XB recruitment, ER, which is sensitive to changes in XB-based cooperativity (Campbell et al., 2004; Campbell and Chandra, 2006; Stelzer et al., 2006; Gollapudi et al., 2017). TnTF88L significantly increases ER at short SL (39%) but not at long SL, suggesting that TnTF88L imparts a greater effect on XB-based cooperativity at short SL. Just as it did in tension experiments, this saturation of XB-based cooperativity at short SL blunted the SL-mediated effects on γ (Fig. 4) and ER (Fig. 5) in TnTF88L fibers.
With respect to XB-based feedback effects (XB–XB and XB–RU cooperativity) and changes in Ca2+ sensitivity, the consequence of augmented XB–RU cooperativity is of importance, because changes in XB–RU cooperativity are most prominently reflected by changes in Ca2+ sensitivity (Razumova et al., 2000). In this context, a consequence of increased XB–RU cooperativity at short SL is that it engages more RUs in XB–RU population, causing the depletion of RU pool and limiting the scope of XB–RU population to increase as SL increases. Therefore, lack of SL-mediated increase in XB–RU cooperativity in TnTF88L fibers would explain why an increase in SL results in no further increase in myofilament Ca2+ sensitivity (Fig. 2 C). Although we cannot definitively distinguish between the two types of XB-based cooperativity, findings from previous modeling simulations (Razumova et al., 2000)—in conjunction with data from our study—provide clues about the effect of TnTF88L on XB-based cooperativity. Previous modeling studies have suggested that changes in XB–XB cooperativity have an impact on maximal tension, ktr, and pCa50 such that an increase in XB–XB cooperativity augments maximal tension, slows ktr, and decreases pCa50 (and vice versa; Razumova et al., 2000). In this context, our observation that TnTF88L does not alter maximal tension and ktr, and significantly increases pCa50, allows us to speculate that TnTF88L may have little to no impact on XB–XB cooperativity.
Conclusion
TnTF88L increases myofilament Ca2+ sensitivity at both short and long SLs; however, the effect is disproportionately greater at short SL, which results in abolishment of length-dependent activation. Under submaximal activating conditions, increased myofilament Ca2+ sensitivity, combined with an increase in ER, may increase ventricular force output during systole and prolong systolic ejection time (Davis et al., 2001; Stelzer et al., 2006; Stelzer and Moss, 2006), leading to a delay in ventricular relaxation. Slowed ventricular relaxation would offer greater resistance to ventricular filling in late diastole such that ventricular diastolic pressure is elevated; this may lead to dyspnea, as observed in patients harboring F87L (Gimeno et al., 2009). Impairment of length-dependent activation suggests that the Frank–Starling mechanism may be significantly impaired in intact hearts containing F87L. Under increased hemodynamic demands, attenuation of the Frank–Starling mechanism may have severe consequences on heart function because it limits the ability of the heart to increase its stroke volume in response to an increase in venous return. In some cases, however, a substantial increase in myofilament Ca2+ sensitivity and subsequent increase in myocardial force development may offer a benefit to F87L patients who are at advanced stages of heart failure. Patients at advanced stages of heart failure are likely to minimize high stress workloads and thus operate at lower end-diastolic volumes; under such conditions, increased force at short SL may improve contraction.
Supplementary Material
Acknowledgments
This work was supported, in part, by the National Institutes of Health (HL-075643 to M. Chandra).
The authors declare no competing financial interests. This paper does not contain clinical studies of patient data.
Author contributions: S.M. Reda: study conception, design, data acquisition, data analysis, data interpretation, and drafting of the manuscript. M. Chandra: study conception, design, data interpretation, and drafting of the manuscript.
Henk L. Granzier served as editor.
References
- Abraham D.M., Davis R.T. III, Warren C.M., Mao L., Wolska B.M., Solaro R.J., and Rockman H.A.. 2016. β-Arrestin mediates the Frank-Starling mechanism of cardiac contractility. Proc. Natl. Acad. Sci. USA. 113:14426–14431. 10.1073/pnas.1609308113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D.G., and Kentish J.C.. 1985. The cellular basis of the length-tension relation in cardiac muscle. J. Mol. Cell. Cardiol. 17:821–840. 10.1016/S0022-2828(85)80097-3 [DOI] [PubMed] [Google Scholar]
- Arteaga G.M., Palmiter K.A., Leiden J.M., and Solaro R.J.. 2000. Attenuation of length dependence of calcium activation in myofilaments of transgenic mouse hearts expressing slow skeletal troponin I. J. Physiol. 526:541–549. 10.1111/j.1469-7793.2000.t01-1-00541.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., and Eisenberg E.. 1986. Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc. Natl. Acad. Sci. USA. 83:3542–3546. 10.1073/pnas.83.10.3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. 1997. Rate constant of muscle force redevelopment reflects cooperative activation as well as cross-bridge kinetics. Biophys. J. 72:254–262. 10.1016/S0006-3495(97)78664-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K.B., and Chandra M.. 2006. Functions of stretch activation in heart muscle. J. Gen. Physiol. 127:89–94. 10.1085/jgp.200509483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K.B., Chandra M., Kirkpatrick R.D., Slinker B.K., and Hunter W.C.. 2004. Interpreting cardiac muscle force-length dynamics using a novel functional model. Am. J. Physiol. Heart Circ. Physiol. 286:H1535–H1545. 10.1152/ajpheart.01029.2003 [DOI] [PubMed] [Google Scholar]
- Chandra M., Kim J.J., and Solaro R.J.. 1999. An improved method for exchanging troponin subunits in detergent skinned rat cardiac fiber bundles. Biochem. Biophys. Res. Commun. 263:219–223. 10.1006/bbrc.1999.1341 [DOI] [PubMed] [Google Scholar]
- Chandra M., Tschirgi M.L., and Tardiff J.C.. 2005. Increase in tension-dependent ATP consumption induced by cardiac troponin T mutation. Am. J. Physiol. Heart Circ. Physiol. 289:H2112–H2119. 10.1152/ajpheart.00571.2005 [DOI] [PubMed] [Google Scholar]
- Chandra M., Tschirgi M.L., Rajapakse I., and Campbell K.B.. 2006. Troponin T modulates sarcomere length-dependent recruitment of cross-bridges in cardiac muscle. Biophys. J. 90:2867–2876. 10.1529/biophysj.105.076950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra V., Gollapudi S.K., and Chandra M.. 2015. Rat cardiac troponin T mutation (F72L)-mediated impact on thin filament cooperativity is divergently modulated by α- and β-myosin heavy chain isoforms. Am. J. Physiol. Heart Circ. Physiol. 309:H1260–H1270. 10.1152/ajpheart.00519.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J.S., Hassanzadeh S., Winitsky S., Lin H., Satorius C., Vemuri R., Aletras A.H., Wen H., and Epstein N.D.. 2001. The overall pattern of cardiac contraction depends on a spatial gradient of myosin regulatory light chain phosphorylation. Cell. 107:631–641. 10.1016/S0092-8674(01)00586-4 [DOI] [PubMed] [Google Scholar]
- de Tombe P.P., and Stienen G.J.. 1995. Protein kinase A does not alter economy of force maintenance in skinned rat cardiac trabeculae. Circ. Res. 76:734–741. 10.1161/01.RES.76.5.734 [DOI] [PubMed] [Google Scholar]
- Fitzsimons D.P., and Moss R.L.. 1998. Strong binding of myosin modulates length-dependent Ca2+ activation of rat ventricular myocytes. Circ. Res. 83:602–607. 10.1161/01.RES.83.6.602 [DOI] [PubMed] [Google Scholar]
- Ford S.J., and Chandra M.. 2013. Length-dependent effects on cardiac contractile dynamics are different in cardiac muscle containing α- or β-myosin heavy chain. Arch. Biochem. Biophys. 535:3–13. 10.1016/j.abb.2012.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford S.J., Chandra M., Mamidi R., Dong W., and Campbell K.B.. 2010. Model representation of the nonlinear step response in cardiac muscle. J. Gen. Physiol. 136:159–177. 10.1085/jgp.201010467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford S.J., Mamidi R., Jimenez J., Tardiff J.C., and Chandra M.. 2012. Effects of R92 mutations in mouse cardiac troponin T are influenced by changes in myosin heavy chain isoform. J. Mol. Cell. Cardiol. 53:542–551. 10.1016/j.yjmcc.2012.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadharan B., Sunitha M.S., Mukherjee S., Chowdhury R.R., Haque F., Sekar N., Sowdhamini R., Spudich J.A., and Mercer J.A.. 2017. Molecular mechanisms and structural features of cardiomyopathy-causing troponin T mutants in the tropomyosin overlap region. Proc. Natl. Acad. Sci. USA. 114:11115–11120. 10.1073/pnas.1710354114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno J.R., Monserrat L., Pérez-Sánchez I., Marín F., Caballero L., Hermida-Prieto M., Castro A., and Valdés M.. 2009. Hypertrophic cardiomyopathy. A study of the troponin-T gene in 127 Spanish families. Rev. Esp. Cardiol. 62:1473–1477. 10.1016/S0300-8932(09)73136-7 [DOI] [PubMed] [Google Scholar]
- Gollapudi S.K., and Chandra M.. 2016a Dilated cardiomyopathy mutation (R134W) in mouse cardiac troponin T induces greater contractile deficits against α-myosin heavy chain than against β-myosin heavy chain. Front. Physiol. 7:443 10.3389/fphys.2016.00443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollapudi S.K., and Chandra M.. 2016b The effect of cardiomyopathy mutation (R97L) in mouse cardiac troponin T on the muscle length-mediated recruitment of crossbridges is modified divergently by α- and β-myosin heavy chain. Arch. Biochem. Biophys. 601:105–112. 10.1016/j.abb.2016.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollapudi S.K., Mamidi R., Mallampalli S.L., and Chandra M.. 2012. The N-terminal extension of cardiac troponin T stabilizes the blocked state of cardiac thin filament. Biophys. J. 103:940–948. 10.1016/j.bpj.2012.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollapudi S.K., Gallon C.E., and Chandra M.. 2013. The tropomyosin binding region of cardiac troponin T modulates crossbridge recruitment dynamics in rat cardiac muscle fibers. J. Mol. Biol. 425:1565–1581. 10.1016/j.jmb.2013.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollapudi S.K., Tardiff J.C., and Chandra M.. 2015. The functional effect of dilated cardiomyopathy mutation (R144W) in mouse cardiac troponin T is differently affected by α- and β-myosin heavy chain isoforms. Am. J. Physiol. Heart Circ. Physiol. 308:H884–H893. 10.1152/ajpheart.00528.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollapudi S.K., Reda S.M., and Chandra M.. 2017. Omecamtiv mecarbil abolishes length-mediated increase in guinea pig cardiac myofiber Ca2+ sensitivity. Biophys. J. 113:880–888. 10.1016/j.bpj.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeley D.H., Golosinska K., and Smillie L.B.. 1987. The effects of troponin T fragments T1 and T2 on the binding of nonpolymerizable tropomyosin to F-actin in the presence and absence of troponin I and troponin C. J. Biol. Chem. 262:9971–9978. [PubMed] [Google Scholar]
- Hinkle A., and Tobacman L.S.. 2003. Folding and function of the troponin tail domain. Effects of cardiomyopathic troponin T mutations. J. Biol. Chem. 278:506–513. 10.1074/jbc.M209194200 [DOI] [PubMed] [Google Scholar]
- Holubarsch C., Ruf T., Goldstein D.J., Ashton R.C., Nickl W., Pieske B., Pioch K., Lüdemann J., Wiesner S., Hasenfuss G., et al. 1996. Existence of the Frank-Starling mechanism in the failing human heart. Investigations on the organ, tissue, and sarcomere levels. Circulation. 94:683–689. 10.1161/01.CIR.94.4.683 [DOI] [PubMed] [Google Scholar]
- Jackson P., Amphlett G.W., and Perry S.V.. 1975. The primary structure of troponin T and the interaction with tropomyosin. Biochem. J. 151:85–97. 10.1042/bj1510085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobirumaki-Shimozawa F., Inoue T., Shintani S.A., Oyama K., Terui T., Minamisawa S., Ishiwata S., and Fukuda N.. 2014. Cardiac thin filament regulation and the Frank-Starling mechanism. J. Physiol. Sci. 64:221–232. 10.1007/s12576-014-0314-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konhilas J.P., Irving T.C., and de Tombe P.P.. 2002a Frank-Starling law of the heart and the cellular mechanisms of length-dependent activation. Pflugers Arch. 445:305–310. 10.1007/s00424-002-0902-1 [DOI] [PubMed] [Google Scholar]
- Konhilas J.P., Irving T.C., and de Tombe P.P.. 2002b Length-dependent activation in three striated muscle types of the rat. J. Physiol. 544:225–236. 10.1113/jphysiol.2002.024505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte F.S., and McDonald K.S.. 2007. Sarcomere length dependence of rat skinned cardiac myocyte mechanical properties: dependence on myosin heavy chain. J. Physiol. 581:725–739. 10.1113/jphysiol.2007.128199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer S.S., and Geeves M.A.. 1998. The muscle thin filament as a classical cooperative/allosteric regulatory system. J. Mol. Biol. 277:1081–1089. 10.1006/jmbi.1998.1654 [DOI] [PubMed] [Google Scholar]
- Liu B., Tikunova S.B., Kline K.P., Siddiqui J.K., and Davis J.P.. 2012. Disease-related cardiac troponins alter thin filament Ca2+ association and dissociation rates. PLoS One. 7:e38259 10.1371/journal.pone.0038259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamidi R., Mallampalli S.L., Wieczorek D.F., and Chandra M.. 2013. Identification of two new regions in the N-terminus of cardiac troponin T that have divergent effects on cardiac contractile function. J. Physiol. 591:1217–1234. 10.1113/jphysiol.2012.243394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery D.E., Tardiff J.C., and Chandra M.. 2001. Cardiac troponin T mutations: correlation between the type of mutation and the nature of myofilament dysfunction in transgenic mice. J. Physiol. 536:583–592. 10.1111/j.1469-7793.2001.0583c.xd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R.L., and Fitzsimons D.P.. 2002. Frank-Starling relationship: long on importance, short on mechanism. Circ. Res. 90:11–13. [PubMed] [Google Scholar]
- Narolska N.A., Eiras S., van Loon R.B., Boontje N.M., Zaremba R., Spiegelen Berg S.R., Stooker W., Huybregts M.A., Visser F.C., van der Velden J., and Stienen G.J.. 2005a Myosin heavy chain composition and the economy of contraction in healthy and diseased human myocardium. J. Muscle Res. Cell Motil. 26:39–48. 10.1007/s10974-005-9005-x [DOI] [PubMed] [Google Scholar]
- Narolska N.A., van Loon R.B., Boontje N.M., Zaremba R., Penas S.E., Russell J., Spiegelenberg S.R., Huybregts M.A., Visser F.C., de Jong J.W., et al. 2005b Myocardial contraction is 5-fold more economical in ventricular than in atrial human tissue. Cardiovasc. Res. 65:221–229. 10.1016/j.cardiores.2004.09.029 [DOI] [PubMed] [Google Scholar]
- Nowak G., Peña J.R., Urboniene D., Geenen D.L., Solaro R.J., and Wolska B.M.. 2007. Correlations between alterations in length-dependent Ca2+ activation of cardiac myofilaments and the end-systolic pressure-volume relation. J. Muscle Res. Cell Motil. 28:415–419. 10.1007/s10974-008-9136-y [DOI] [PubMed] [Google Scholar]
- Palm T., Graboski S., Hitchcock-DeGregori S.E., and Greenfield N.J.. 2001. Disease-causing mutations in cardiac troponin T: identification of a critical tropomyosin-binding region. Biophys. J. 81:2827–2837. 10.1016/S0006-3495(01)75924-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnick G.D., Becker L.C., Fisher M.L., Gerstenblith G., Renlund D.G., Fleg J.L., Weisfeldt M.L., and Lakatta E.G.. 1986. Use of the Frank-Starling mechanism during submaximal versus maximal upright exercise. Am. J. Physiol. 251:H1101–H1105. [DOI] [PubMed] [Google Scholar]
- Razumova M.V., Bukatina A.E., and Campbell K.B.. 2000. Different myofilament nearest-neighbor interactions have distinctive effects on contractile behavior. Biophys. J. 78:3120–3137. 10.1016/S0006-3495(00)76849-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda S.M., Gollapudi S.K., and Chandra M.. 2016. L71F mutation in rat cardiac troponin T augments crossbridge recruitment and detachment dynamics against α-myosin heavy chain, but not against β-myosin heavy chain. J. Muscle Res. Cell Motil. 37:215–223. 10.1007/s10974-016-9460-6 [DOI] [PubMed] [Google Scholar]
- Schwinger R.H., Böhm M., Koch A., Schmidt U., Morano I., Eissner H.J., Uberfuhr P., Reichart B., and Erdmann E.. 1994. The failing human heart is unable to use the Frank-Starling mechanism. Circ. Res. 74:959–969. 10.1161/01.RES.74.5.959 [DOI] [PubMed] [Google Scholar]
- Sequeira V., Wijnker P.J., Nijenkamp L.L., Kuster D.W., Najafi A., Witjas-Paalberends E.R., Regan J.A., Boontje N., Ten Cate F.J., Germans T., et al. 2013. Perturbed length-dependent activation in human hypertrophic cardiomyopathy with missense sarcomeric gene mutations. Circ. Res. 112:1491–1505. 10.1161/CIRCRESAHA.111.300436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L., Tainter C., Regnier M., and Martyn D.A.. 2009. Cooperative cross-bridge activation of thin filaments contributes to the Frank-Starling mechanism in cardiac muscle. Biophys. J. 96:3692–3702. 10.1016/j.bpj.2009.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommese R.F., Nag S., Sutton S., Miller S.M., Spudich J.A., and Ruppel K.M.. 2013. Effects of troponin T cardiomyopathy mutations on the calcium sensitivity of the regulated thin filament and the actomyosin cross-bridge kinetics of human β-cardiac myosin. PLoS One. 8:e83403 10.1371/journal.pone.0083403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer J.E., and Moss R.L.. 2006. Contributions of stretch activation to length-dependent contraction in murine myocardium. J. Gen. Physiol. 128:461–471. 10.1085/jgp.200609634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer J.E., Larsson L., Fitzsimons D.P., and Moss R.L.. 2006. Activation dependence of stretch activation in mouse skinned myocardium: implications for ventricular function. J. Gen. Physiol. 127:95–107. 10.1085/jgp.200509432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienen G.J., Zaremba R., and Elzinga G.. 1995. ATP utilization for calcium uptake and force production in skinned muscle fibres of Xenopus laevis. J. Physiol. 482:109–122. 10.1113/jphysiol.1995.sp020503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardiff J.C., Factor S.M., Tompkins B.D., Hewett T.E., Palmer B.M., Moore R.L., Schwartz S., Robbins J., and Leinwand L.A.. 1998. A truncated cardiac troponin T molecule in transgenic mice suggests multiple cellular mechanisms for familial hypertrophic cardiomyopathy. J. Clin. Invest. 101:2800–2811. 10.1172/JCI2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velden J., Moorman A.F., and Stienen G.J.. 1998. Age-dependent changes in myosin composition correlate with enhanced economy of contraction in guinea-pig hearts. J. Physiol. 507:497–510. 10.1111/j.1469-7793.1998.497bt.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk S.J., Paalberends E.R., Najafi A., Michels M., Sadayappan S., Carrier L., Boontje N.M., Kuster D.W., van Slegtenhorst M., Dooijes D., et al. 2012. Contractile dysfunction irrespective of the mutant protein in human hypertrophic cardiomyopathy with normal systolic function. Circ Heart Fail. 5:36–46. 10.1161/CIRCHEARTFAILURE.111.963702 [DOI] [PubMed] [Google Scholar]
- Wang Y.P., and Fuchs F.. 1994. Length, force, and Ca(2+)-troponin C affinity in cardiac and slow skeletal muscle. Am. J. Physiol. 266:C1077–C1082. 10.1152/ajpcell.1994.266.4.C1077 [DOI] [PubMed] [Google Scholar]
- Williams M.R., Lehman S.J., Tardiff J.C., and Schwartz S.D.. 2016. Atomic resolution probe for allostery in the regulatory thin filament. Proc. Natl. Acad. Sci. USA. 113:3257–3262. 10.1073/pnas.1519541113 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.