Abstract
Background
The incidence of gastric cancer in Asia is higher than that in Europe and Northern America. Helicobacter pylori infection is the most important factor for the development of atrophic gastritis and gastric cancer. The geographical distribution of the prevalence and virulence factors of H. pylori are important to understand the difference between gastritis in the East and West.
Summary
Articles comparing gastritis cases between eastern and western countries showed that the severity of gastritis is closely related to the risk of gastric cancer, and the severity of gastritis is more advanced in East Asia. Although the prevalence of H. pylori infection is closely associated with the incidence of gastric cancer in European countries, the severity of gastritis and the high incidence of gastric cancer in East Asia are not dependent only on the prevalence of H. pylori infection itself. From the viewpoint of the virulence factors of H. pylori, the East Asian CagA-positive strain (EPIYA motif ABD type) is peculiar in East Asia. Considering comprehensively the geographical distribution of H. pylori subtypes is the most important factor among all prospected risk factors for the incidence of gastric cancer and the rate of development of gastritis. While eating habits, such as salty foods, vegetables and fruits, might influence the progression of gastritis, such factors might be responsible for the geographic heterogeneity of gastritis.
Key Message
East Asian CagA-positive H. pylori is the strongest risk factor for gastric carcinogenesis and the development of gastritis.
Key Words: East and West, Gastric cancer, Gastritis, Geographical distribution, Helicobacter pylori infection
Introduction
Gastric cancer is one of the most common causes of cancer-related deaths globally. Herein, Helicobacter pylori infection is the most important factor for the development of atrophic gastritis and gastric cancer [1, 2]. By understanding the geography of chronic atrophic gastritis and gastric cancer, we could develop a method to prevent gastric cancer.
The Geographic Significance of H. pylori Infection, Chronic Gastritis, and Gastric Cancer
The geographic distribution of gastric cancer cases and their incidence is shown in table 1[3]. From the global scale, a higher incidence of gastric cancer is found in Asia than in Europe and Northern America. In East Asia, the incidence rate of gastric cancer is very high. Among European countries, the incidence of gastric cancer in Central, Eastern, and Southern Europe is higher than that in Northern and Western Europe.
Table 1.
The geography of the incidence of gastric cancer
| Population | Total incidence, n | ASR (W) | Cumulative risk (0–74 years) | Prevalence of H. pylori infection, % |
|---|---|---|---|---|
| World | 951,594 | 12.1 | 1.39 | |
| Asia | 699,954 | 15.8 | 1.77 | |
| Eastern Asia | 552,935 | 24.2 | 2.71 | |
| China | 404,996 | 22.7 | 2.51 | 43.8–47.0 |
| Japan | 107,898 | 29.9 | 3.52 | 27.5–35.1 |
| Korea | 31,269 | 41.8 | 4.95 | 54.4 |
| Southeastern Asia | 33,572 | 6.0 | 0.70 | |
| Thailand | 2,841 | 3.1 | 0.35 | 45.9 |
| Vietnam | 14,203 | 16.3 | 1.89 | 65.6 |
| South-Central Asia | 96,288 | 6.7 | 0.79 | |
| India | 63,097 | 6.1 | 0.73 | 79.0 |
| Europe | 139,667 | 9.4 | 1.11 | |
| Central and Eastern Europe | 69,651 | 13.5 | 1.65 | |
| Russian Federation | 38,417 | 16.0 | 1.97 | 90.4 |
| Northern Europe | 11,664 | 5.4 | 0.61 | |
| UK | 6,684 | 4.7 | 0.52 | 14.0 |
| Latvia | 640 | 14.3 | 1.72 | 65.5 |
| Lithuania | 867 | 13.8 | 1.65 | 69.7 |
| Southern Europe | 30,385 | 8.6 | 0.98 | |
| Italy | 13,001 | 8.2 | 0.91 | 33.9 |
| Spain | 7,810 | 7.8 | 0.89 | 60.3 |
| Western Europe | 27,967 | 6.3 | 0.73 | |
| Germany | 16,015 | 7.8 | 0.89 | 48.0 |
| Switzerland | 683 | 4.2 | 0.47 | 11.9 |
| Northern America | 24,502 | 4.0 | 0.46 | |
| USA | 21,155 | 3.9 | 0.45 | 9.6 |
ASR (W) = Estimated age-standardized rates (World) per 100,000.
Recently, a large-scale study reported that the rate of H. pylori infection in Japan is 27.5% [4]. On the other hand, Kamada et al. [5] showed a progressive and rapid decline in the prevalence of H. pylori infection. The overall prevalence of H. pylori infection decreased significantly from 74.7% (1970s) to 53% (1990s) and 35.1% (2010s) [5]. In Korea, a large-scale study showed that the prevalence of H. pylori infection was 54.4% and that it decreased from 66.9% (1998) to 59.6% (2005) and 54.4% (2013) [6]. In China, the prevalence of H. pylori infection was reported as 43.8–47.0% [7, 8]. Moreover, similar to Japan and Korea, Chen et al. [8] showed that the prevalence of H. pylori infection is decreasing in China. Among Southeastern and South Asian countries, the reported prevalence rate of H. pylori infection was 45.9% in Thailand [9], 65.6% in Vietnam [10], and 79.0% in India [11]. In the USA, the H. pylori prevalence among the majority of Americans was 9.6% [12]. Among European countries, the prevalence of H. pylori infection is 90.4% in the Russian Federation [13], 14.0% in the UK [14], 33.9% in Italy [15], 60.3% in Spain [16], 48.0% in Germany [17], and 11.9% in Switzerland [18]. The geographical distribution of the prevalence of H. pylori infection in Europe is heterogeneous. The decrease in the prevalence of H. pylori infection has been confirmed in Russia and Germany [17, 19]. These data revealed that the prevalence of H. pylori infection is closely associated with the incidence of gastric cancer in European countries, but the high incidence of gastric cancer in East Asia is not explained by the prevalence of H. pylori infection alone.
Chronic atrophic gastritis, mainly induced by H. pylori, is strongly associated with gastric cancer [20, 21]. Understanding the geographical significance of chronic gastritis is important to predict the risk of gastric cancer. However, to our knowledge, chronic atrophic gastritis has rarely been compared between different countries. Liu et al. [22] evaluated H. pylori gastritis from seven countries (China, Thailand, Portugal, the Netherlands, Japan, Finland, and Germany) by using the updated Sydney system [23]. Among these countries, the highest scores for antral atrophy were found in Japan. Slightly lower scores were found in China, and the lowest scores were found in Thailand and in European countries such as Finland, the Netherlands, Germany, and Portugal. They also showed that the prevalence of antral atrophy significantly correlated with the incidence of gastric cancer [22]. Naylor et al. [24] performed a cross sectional comparative study on chronic gastritis between the UK and Japan by using the updated Sydney system [23]. In the patients with chronic gastritis, there was no significant difference in the prevalence of H. pylori infection between the UK (89.8%) and Japan (85.5%). However, the atrophy, intestinal metaplasia, and inflammation scores in Japan were much higher than those in the UK. Interestingly, they also showed that the proportion of a corpus predominant or pangastritis in Japanese patients was high, while that of an antral predominant gastritis in UK patients was high. El-Zimaity et al. [25] evaluated mapped gastric biopsy specimens from patients with duodenal ulcer in four countries (Korea, Colombia, USA, and South Africa) for H. pylori infection, active inflammation, and intestinal metaplasia. In their study, no differences were observed in the H. pylori infection rate among these countries. The prevalence of intestinal metaplasia was significantly greater in Korean patients (86%) than that in other countries (50%). Jonaitis et al. [26] compared the prevalence and severity of gastric atrophy between Eastern European (Lithuania and Latvia) and Asian (Taiwan) countries in patients with dyspeptic symptomsby using the updated Sydney system [23]. H. pylori positivity was estimated as 82.7% in Taiwanese patients, 65.5% in Latvian patients, and 69.7% in Lithuanian patients. In patients with H. pylori infection, there were no significant differences in the prevalence of gastric mucosal atrophy and intestinal metaplasia among the countries [26]. Based on the interpretation of the data in table 1, it is important to pay attention to the higher incidence rate of gastric cancer in Lithuania and Latvia among European countries, which is rather close to that in East Asia. These reports suggested that the risk of gastric cancer would be strongly correlated with the severity of atrophic gastritis and intestinal metaplasia rather than only with the difference in geography. On the other hand, it is also true that there are geographic differences in the distribution of atrophic gastritis and intestinal metaplasia induced by H. pylori [22, 24, 25]. Against this background, we review the risk factors of gastric cancer from the subtypes of H. pylori and from the genetics of the host side.
The Geographic Significance of H. pylori Subtypes
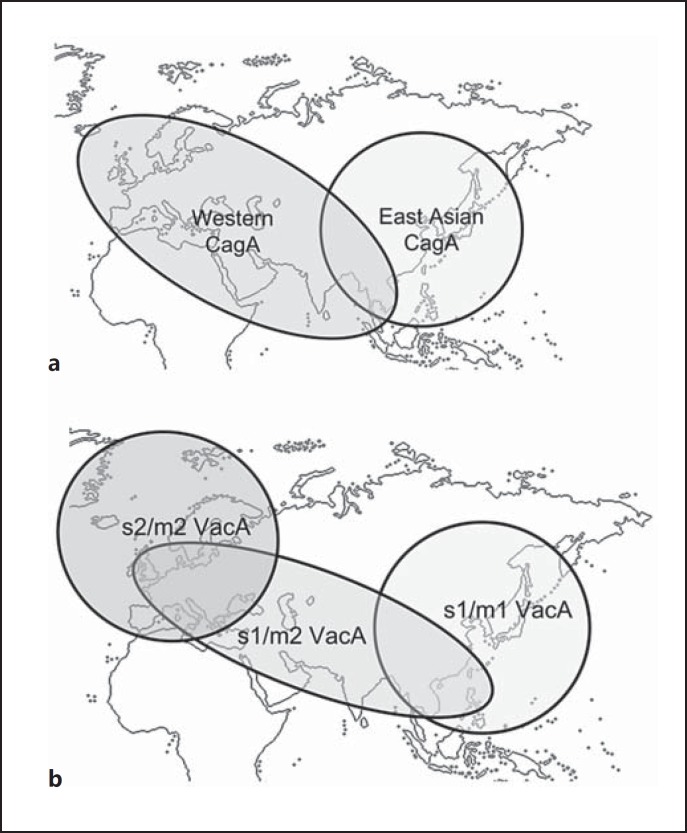
Several H. pylori virulence genes have been well studied and established. Of the virulence factors, cytotoxin-associated gene A (CagA) and vacuolating cytotoxin A (VacA) are the major pathogenic factors. CagA protein of H. pylori, which is delivered into the gastric epithelial cells via bacterial type IV secretion, is an oncoprotein that can induce malignant neoplasms [27, 28]. The phosphorylation motifs are defined by the Glu-Pro-Ile-Tyr-Ala (EPIYA) sequence and are classified as EPIYA-A, B, C, or D according to the amino acids that flank these motifs, and then CagA isoforms with segments C and D are related to Western and East Asian countries, respectively [29, 30]. It suggests that H. pylori is classified into three types of strains: the East Asian CagA-positive strain (EPIYA motif ABD type), the western CagA-positive strain (EPIYA motif ABC type), and the CagA-negative strain. Azuma [30] investigated the distribution of CagA protein diversity in the world by using the data deposited in the GenBank. Interestingly, all western strains (from Ireland, Austria, Italy, England, United States, and Australia) had western CagA. In contrast, all East Asian strains (from Japan, Korea, and China) had East Asian CagA. Among Southeastern and South Asian countries, all strains in Vietnam had East Asian CagA, all strains in India had western CagA, and the strains in Thailand had both East Asian and western CagA (fig. 1a). As shown in table 1, it is clear that the incidence of gastric cancer in Vietnam is higher than that in Thailand and India. Unfortunately, there is no available data about CagA subtypes in the Baltic States, which have a relatively high gastric cancer incidence in Europe.
Fig. 1.
a A global distribution map of CagA subtypes. b A global distribution map of VacA subtypes.
All strains of H. pylori possess the VacA gene, and more than half of these strains express this gene [31]. VacA induces intracytoplasmic vacuolization in epithelial cells, cytochrome c release from mitochondria progressing to apoptosis, and autophagosome formation for inducing autophagy [28, 32, 33]. Sugimoto and colleagues [34, 35] reported that the VacA genotypes with s1 and/or m1 significantly increased the risk of gastric cancer and peptic ulcers. Moreover, the CagA-positive genotype frequently coincided with s1 and m1 genotypes in both populations in the Middle East, Africa, and western countries (fig. 1b). Do Carmo and Rabenhorst [36] reported that the s1-VacA genotype is related to the incidence of gastric cancer among CagA-negative strains in Brazil [36]. Figura et al. [37] showed that 80% of the s1/m2 VacA strains were isolated from Italian patients with diffuse gastric cancer, and suggested that s1/m2 VacA strains might affect histological variety of cancer. Yamaoka et al. [38] showed the geographical significance of the VacA genotypes. According to their study, the strains of Japan and Korea had almost the s1/m1 VacA genotype, while those of Taiwan and Vietnam had the s1/m1 or s1/m2 VacA genotypes, and those of European countries had the s1/m2 or s2/m2 VacA genotypes. On the other hand, Rhead et al. [39] determined that the VacA i1-type was independently associated with gastric cancer development in the western countries. Basiri et al. [40] suggested that the VacA d1-type might predict the risk for gastric cancer development in East Azerbaijan, Iran.
The high incidence of gastric cancer in East Asia indicates the importance of CagA subtypes. On the other hand, the second most important factor from the genetics of H. pylori might be VacA subtypes.
Host Genetic Risk Factors of Gastric Cancer
We must consider the host genetic factors together with the factors from H. pylori for gastric carcinogenesis. Several studies have reported that interleukin-1 polymorphisms are associated with increased risk of gastric cancer in Korea, India, Portugal, and the USA [41, 42, 43, 44, 45]. Other reports showed that the polymorphism in the interleukin-8 and -10 and TNF-α gene is related to the risk of gastric cancer in Korea, the USA, and Portugal, respectively [45, 46, 47].
Diet is also one of the important risk factor of gastric carcinogenesis. A meta-analysis showed an incrementally increasing risk of gastric cancer with higher salt intake [48]. Kamada et al. [5] showed a relationship between high salt intake and the severity of gastritis in Japan. Considering these results, high salt intake might influence gastric carcinogenesis via the development of gastritis. On the other hand, a large prospective European study showed that fruit and vegetable intake were less likely to prevent the development of gastric cancer [49]. A meta-analysis revealed that the consumption of fruit but not of that of vegetables provided a significant protective effect against gastric cancer [50]. Although the bioactive constituents from fruit and/or vegetables, such as selenium, beta-carotene, and vitamins A, C, and E are suggested to protect from various inflammatory damage [51], further studies, such as those on the molecular effect of the prevention of gastritis, are needed.
Conclusions
In this article, we showed the difference in the pathophysiology of gastritis and gastric carcinogenesis in East and West from the viewpoint of the geography and genetics of H. pylori infection as well as host (human) genetic factors. Since 2013, eradication therapy for all H. pylori-associated gastritis has been approved by the Japanese national health insurance system [52]. Moreover, in Japan, the severity of atrophy and intestinal metaplasia also declined remarkably in recent years with the westernization and improvements in economic and hygienic conditions [5]. The incidence of H. pylori infection is steadily declining in developed countries [53]. Various factors, such as geographical and racial significance of H. pylori (infection rate and genetics), diversity of eating habits, and the level of recommendation for H. pylori eradication in each country might affect the geographic heterogeneity of gastritis.
Facts from East and West
Although few East-West comparative studies have been done, the available data suggest that patients from East Asian countries show more severe endoscopic and histologic manifestations of CAG, compared to those from Europe or North America.
In both Asian and western countries, the prevalence of H. pylori infection is linked to the incidence of gastric cancer. Asian countries, however, exhibit a higher incidence of gastric cancer.
Analysis of the H. pylori virulence genes points towards a central role of the CagA and VacA virulence factors. The H. pylori strains may be classified into three types: East Asian CagA-positive (EPIYA motif ABD type), western CagA-positive (EPIYA motif ABC type), and CagA-negative. In particular, the East Asian ABD-type CagA strain is associated with higher pathogenicity. The presence of the s1 and/or m2 VacA genotypes is associated with a greater risk of gastric cancer and peptic ulcers.
The individual's genetic background, including polymorphisms in several interleukin genes, TNF-α, NOD1 and NOD2, may affect the disease risk. Some Asian populations express point mutations in the sej allele, which may facilitate bacterial attachment to the gastric mucosa. Other host factors that influence disease risk are parietal cell mass and ABH-secretor and/or Lewis histo-blood group status. Asians have been shown to have smaller parietal cell masses than western populations, affecting gastric acid secretion and response to treatment with proton pump inhibitors and histamine-2 receptor antagonists.
Data on the protective effect of specific dietary elements remain conflicting. Soy products are widely used in Asia, but data on their beneficial effects depend on their salt content, fermentation, and the presence of contaminants. Some western studies have indicated that the ingestion of dairy products may have protective effects on CAG and gastric cancer. Consumption of green tea has also been associated with reduced risk of CAG in Japanese populations.
Environmental factors (such as air pollution) could act in concert with lifestyle factors (such as smoking and diet) to modulate an individual's risk of GAC or gastric cancer.
Disclosure Statement
During the last 2 years, H.S. received scholarship funds for the research from Astellas Pharm Inc., Astra-Zeneca K.K., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Zeria Pharmaceutical Co., Ltd. and received service honoraria from Astellas Pharm Inc., Astra-Zeneca K.K., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Zeria Pharmaceutical Co., Ltd.
References
- 1.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 2.Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392–397. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4.Hirayama Y, Kawai T, Otaki J, et al. Prevalence of Helicobacter pylori infection with healthy subjects in Japan. J Gastroenterol Hepatol. 2014;29((suppl 4)):16–19. doi: 10.1111/jgh.12795. [DOI] [PubMed] [Google Scholar]
- 5.Kamada T, Haruma K, Ito M, et al. Time trends in Helicobacter pylori infection and atrophic gastritis over 40 years in Japan. Helicobacter. 2015;20:192–198. doi: 10.1111/hel.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim SH, Kwon JW, Kim N, et al. Prevalence and risk factors of Helicobacter pylori infection in Korea: nationwide multicenter study over 13 years. BMC Gastroenterol. 2013;13:104. doi: 10.1186/1471-230X-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu C, Yan M, Sun Y, et al. Prevalence of Helicobacter pylori infection and its relation with body mass index in a Chinese population. Helicobacter. 2014;19:437–442. doi: 10.1111/hel.12153. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Bu XL, Wang QY, et al. Decreasing seroprevalence of Helicobacter pylori infection during 1993–2003 in Guangzhou, southern China. Helicobacter. 2007;12:164–169. doi: 10.1111/j.1523-5378.2007.00487.x. [DOI] [PubMed] [Google Scholar]
- 9.Uchida T, Miftahussurur M, Pittayanon R, et al. Helicobacter pylori infection in Thailand: a nationwide study of the CagA phenotype. PLoS One. 2015;10:e0136775. doi: 10.1371/journal.pone.0136775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen TL, Uchida T, Tsukamoto Y, et al. Helicobacter pylori infection and gastroduodenal diseases in Vietnam: a cross-sectional, hospital-based study. BMC Gastroenterol. 2010;10:114. doi: 10.1186/1471-230X-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham DY, Adam E, Reddy GT, et al. Seroepidemiology of Helicobacter pylori infection in India. Comparison of developing and developed countries. Dig Dis Sci. 1991;36:1084–1088. doi: 10.1007/BF01297451. [DOI] [PubMed] [Google Scholar]
- 12.Choi CE, Sonnenberg A, Turner K, et al. High prevalence of gastric preneoplastic lesions in East Asians and Hispanics in the USA. Dig Dis Sci. 2015;60:2070–2076. doi: 10.1007/s10620-015-3591-2. [DOI] [PubMed] [Google Scholar]
- 13.Rakhmanin Iu A, Zykova IE, Fedichkina TP, et al. The study of spatial distribution of Helicobacter pylori infection rate in able-bodied population of Moscow in the course of medical examination of the manufacturing contingents (in Russian) Gig Sanit. 2013:79–82. [PubMed] [Google Scholar]
- 14.Vyse AJ, Gay NJ, Hesketh LM, et al. The burden of Helicobacter pylori infection in England and Wales. Epidemiol Infect. 2002;128:411–417. doi: 10.1017/s0950268802006970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zullo A, Esposito G, Ridola L, et al. Prevalence of lesions detected at upper endoscopy: an Italian survey. Eur J Intern Med. 2014;25:772–776. doi: 10.1016/j.ejim.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez Ceballos F, Taxonera Samso C, Garcia Alonso C, et al. Prevalence of Helicobacter pylori infection in the healthy population of Madrid (Spain) (in Spanish) Rev Esp Enferm Dig. 2007;99:497–501. doi: 10.4321/s1130-01082007000900003. [DOI] [PubMed] [Google Scholar]
- 17.Michel A, Pawlita M, Boeing H, et al. Helicobacter pylori antibody patterns in Germany: a cross-sectional population study. Gut Pathog. 2014;6:10. doi: 10.1186/1757-4749-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gruber D, Pohl D, Vavricka S, et al. Swiss tertiary care center experience challenges the age-cohort effect in Helicobacter pylori infection. J Gastrointestin Liver Dis. 2008;17:373–377. [PubMed] [Google Scholar]
- 19.Tkachenko MA, Zhannat NZ, Erman LV, et al. Dramatic changes in the prevalence of Helicobacter pylori infection during childhood: a 10-year follow-up study in Russia. J Pediatr Gastroenterol Nutr. 2007;45:428–432. doi: 10.1097/MPG.0b013e318064589f. [DOI] [PubMed] [Google Scholar]
- 20.Adamu MA, Weck MN, Gao L, et al. Incidence of chronic atrophic gastritis: systematic review and meta-analysis of follow-up studies. Eur J Epidemiol. 2010;25:439–448. doi: 10.1007/s10654-010-9482-0. [DOI] [PubMed] [Google Scholar]
- 21.Jaskiewicz K, Louwrens HD. Chronic atrophic gastritis in a population at risk for gastric carcinoma. Anticancer Res. 1991;11:835–839. [PubMed] [Google Scholar]
- 22.Liu Y, Ponsioen CI, Xiao SD, et al. Geographic pathology of Helicobacter pylori gastritis. Helicobacter. 2005;10:107–113. doi: 10.1111/j.1523-5378.2005.00304.x. [DOI] [PubMed] [Google Scholar]
- 23.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Naylor GM, Gotoda T, Dixon M, et al. Why does Japan have a high incidence of gastric cancer? Comparison of gastritis between UK and Japanese patients. Gut. 2006;55:1545–1552. doi: 10.1136/gut.2005.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Zimaity HMT, Gutierrez O, Kim JG, et al. Geographic differences in the distribution of intestinal metaplasia in duodenal ulcer patients. Am J Gastroenterol. 2001;96:666–672. doi: 10.1111/j.1572-0241.2001.03601.x. [DOI] [PubMed] [Google Scholar]
- 26.Jonaitis L, Ivanauskas A, Janciauskas D, et al. Precancerous gastric conditions in high Helicobacter pylori prevalence areas: comparison between Eastern European (Lithuanian, Latvian) and Asian (Taiwanese) patients. Medicina (Kaunas) 2007;43:623–629. [PubMed] [Google Scholar]
- 27.Hatakeyama M. Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis. Cell Host Microbe. 2014;15:306–316. doi: 10.1016/j.chom.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Tsugawa H, Suzuki H, Saya H, et al. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe. 2012;12:764–777. doi: 10.1016/j.chom.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Higashi H, Tsutsumi R, Fujita A, et al. Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc Natl Acad Sci USA. 2002;99:14428–14433. doi: 10.1073/pnas.222375399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azuma T. Helicobacter pylori CagA protein variation associated with gastric cancer in Asia. J Gastroenterol. 2004;39:97–103. doi: 10.1007/s00535-003-1279-4. [DOI] [PubMed] [Google Scholar]
- 31.Yahiro K, Wada A, Nakayama M, et al. Protein-tyrosine phosphatase alpha, RPTP alpha, is a Helicobacter pylori VacA receptor. J Biol Chem. 2003;278:19183–19189. doi: 10.1074/jbc.M300117200. [DOI] [PubMed] [Google Scholar]
- 32.Gebert B, Fischer W, Haas R. The Helicobacter pylori vacuolating cytotoxin: from cellular vacuolation to immunosuppressive activities. Rev Physiol Biochem Pharmacol. 2004;152:205–220. doi: 10.1007/s10254-004-0027-3. [DOI] [PubMed] [Google Scholar]
- 33.Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7:629–641. doi: 10.1038/nrgastro.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugimoto M, Zali MR, Yamaoka Y. The association of vacA genotypes and Helicobacter pylori-related gastroduodenal diseases in the Middle East. Eur J Clin Microbiol Infect Dis. 2009;28:1227–1236. doi: 10.1007/s10096-009-0772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugimoto M, Yamaoka Y. The association of vacA genotype and Helicobacter pylori-related disease in Latin American and African populations. Clin Microbiol Infect. 2009;15:835–842. doi: 10.1111/j.1469-0691.2009.02769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Do Carmo AP, Rabenhorst SH. Importance of vacAs1 gene in gastric cancer patients infected with cagA-negative Helicobacter pylori. Apmis. 2011;119:485–486. doi: 10.1111/j.1600-0463.2011.02739.x. [DOI] [PubMed] [Google Scholar]
- 37.Figura N, Valassina M, Moretti E, et al. Histological variety of gastric carcinoma and Helicobacter pylori cagA and vacA polymorphism. Eur J Gastroenterol Hepatol. 2015;27:1017–1021. doi: 10.1097/MEG.0000000000000414. [DOI] [PubMed] [Google Scholar]
- 38.Yamaoka Y, Orito E, Mizokami M, et al. Helicobacter pylori in North and South America before Columbus. FEBS Lett. 2002;517:180–184. doi: 10.1016/s0014-5793(02)02617-0. [DOI] [PubMed] [Google Scholar]
- 39.Rhead JL, Letley DP, Mohammadi M, et al. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology. 2007;133:926–936. doi: 10.1053/j.gastro.2007.06.056. [DOI] [PubMed] [Google Scholar]
- 40.Basiri Z, Safaralizadeh R, Bonyadi MJ, et al. Helicobacter pylori vacA d1 genotype predicts risk of gastric adenocarcinoma and peptic ulcers in northwestern Iran. Asian Pac J Cancer Prev. 2014;15:1575–1579. doi: 10.7314/apjcp.2014.15.4.1575. [DOI] [PubMed] [Google Scholar]
- 41.Malaty HM, Kim JG, El-Zimaity HM, et al. High prevalence of duodenal ulcer and gastric cancer in dyspeptic patients in Korea. Scand J Gastroenterol. 1997;32:751–754. doi: 10.3109/00365529708996529. [DOI] [PubMed] [Google Scholar]
- 42.Irtiza S, Samie AU, Ali S, et al. IL-1beta polymorphism and expression associated with decreased risk of gastric carcinoma: a case control study in the ethnic Kashmiri population, India. Asian Pac J Cancer Prev. 2015;16:1987–1992. doi: 10.7314/apjcp.2015.16.5.1987. [DOI] [PubMed] [Google Scholar]
- 43.Figueiredo C, Machado JC, Pharoah P, et al. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst. 2002;94:1680–1687. doi: 10.1093/jnci/94.22.1680. [DOI] [PubMed] [Google Scholar]
- 44.El-Omar EM, Carrington M, Chow WH, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 45.El-Omar EM, Rabkin CS, Gammon MD, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193–201. doi: 10.1016/s0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 46.Machado JC, Figueiredo C, Canedo P, et al. A proinflammatory genetic profile increases the risk for chronic atrophic gastritis and gastric carcinoma. Gastroenterology. 2003;125:364–371. doi: 10.1016/s0016-5085(03)00899-0. [DOI] [PubMed] [Google Scholar]
- 47.Lee KE, Khoi PN, Xia Y, et al. Helicobacter pylori and interleukin-8 in gastric cancer. World J Gastroenterol. 2013;19:8192–8202. doi: 10.3748/wjg.v19.i45.8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D'Elia L, Rossi G, Ippolito R, et al. Habitual salt intake and risk of gastric cancer: a meta-analysis of prospective studies. Clin Nutr. 2012;31:489–498. doi: 10.1016/j.clnu.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez CA, Lujan-Barroso L, Bueno-de-Mesquita HB, et al. Fruit and vegetable intake and the risk of gastric adenocarcinoma: a reanalysis of the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) study after a longer follow-up. Int J Cancer. 2012;131:2910–2919. doi: 10.1002/ijc.27565. [DOI] [PubMed] [Google Scholar]
- 50.Wang Q, Chen Y, Wang X, et al. Consumption of fruit, but not vegetables, may reduce risk of gastric cancer: results from a meta-analysis of cohort studies. Eur J Cancer. 2014;50:1498–1509. doi: 10.1016/j.ejca.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 51.Sjunnesson H, Sturegard E, Willen R, et al. High intake of selenium, beta-carotene, and vitamins A, C, and E reduces growth of Helicobacter pylori in the guinea pig. Comp Med. 2001;51:418–423. [PubMed] [Google Scholar]
- 52.Suzuki H, Mori H. Helicobacter pylori: Helicobacter pylori gastritis – a novel distinct disease entity. Nat Rev Gastroenterol Hepatol. 2015;12:556–557. doi: 10.1038/nrgastro.2015.158. [DOI] [PubMed] [Google Scholar]
- 53.de Korwin JD. Epidemiology of Helicobacter pylori infection and gastric cancer (in French) Rev Prat. 2014;64:189–193. [PubMed] [Google Scholar]