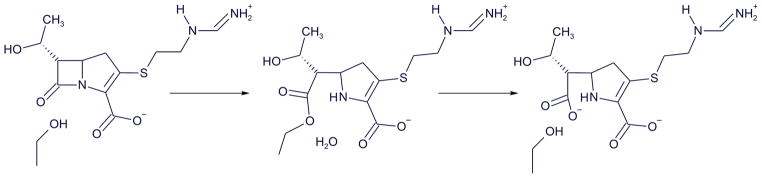
Figure 2. General mechanism for class D β-lactamases.
Class D carbapenemases like OXA-239 use an active site serine to attack the carbonyl carbon on the β-lactam ring, resulting in the drug forming an acyl-intermediate with the serine. This intermediate is subsequently hydrolyzed to release the inactive drug with a broken β-lactam ring.