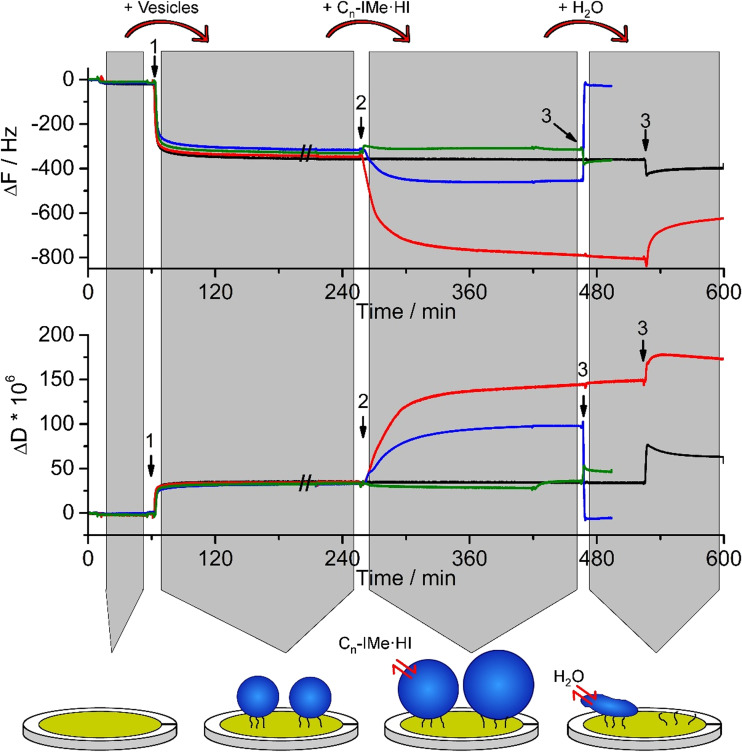
Fig. 5.
Bilayer interactions of Cn-IMe·HI on POPC/POPS (4:1) vesicles probed by QCM-D. Large unilamellar vesicles of POPC/POPS (4:1, 200 nm) were surface-tethered by biotin-streptavidin linkers on a self-assembled monolayer coated sensor surface (arrows 1). Following equilibration with tris-buffered-saline, pH 7.4, suspensions of 0.1 mM (Cn-IMe·HI, n = 7, 11, 15) imidazolium salts were incubated, which induced diverse membrane interactions (arrows 2). Remarkably, the C15 (red) and C11 (blue) compounds lead to an increase in surface associated mass, indicated by ΔΔF and an increased viscoelasticity of the associated layers (ΔΔD), whereas the short chain C7 (green) imidazolium salt did not much alter vesicle membranes. After another equilibration step with buffer for 30–60 min, rinsing with pure water applied hypo-osmotic conditions (arrows 3). The osmotic gradient across the vesicle membrane resulted in a severe loss of vesicle mass after interaction with the C11 compound. Vesicles incubated with the C15 imidazolium salt partially lost some mass and vesicles treated by the C7 compound did not considerably change their mass. As a control, ~0.5 mg/ml POPC:POPS (4:1), 200-nm vesicles were also applied (black). The cartoon represents the effects of imidazolium salt on the vesicles (e.g. C11-IMe·HI). Modified from Drücker et al. (2017) and Wang et al. (2015a, b)