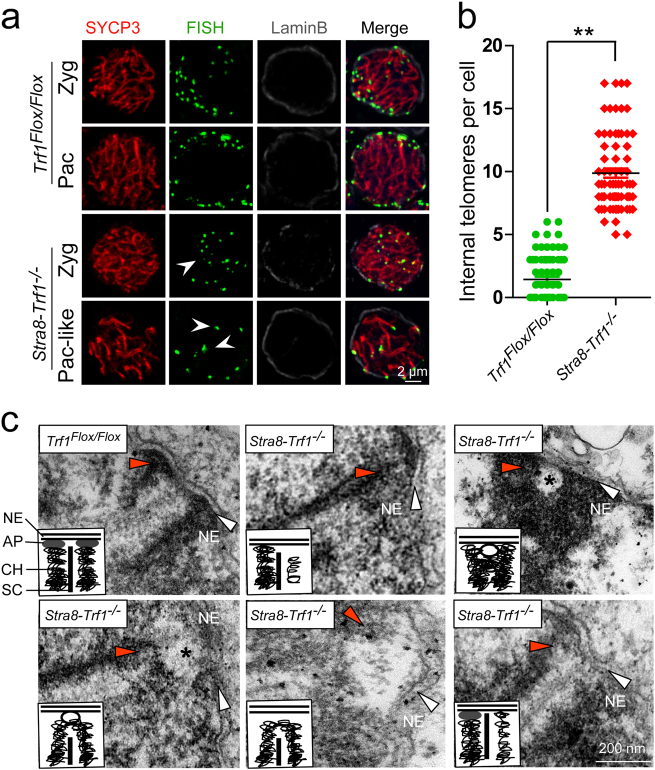
Fig. 3. TRF1 is required for the attachment of telomeres to the nuclear envelope in spermatocytes.
a Increased detachment of telomeres from NE after Trf1 deletion. An increased number of telomeres were distributed at the inner site of nucleus in Stra8-Trf1−/− spermatocytes compared with the control group. Paraffin sections from Trf1Flox/Flox and Stra8-Trf1−/− testes were immunostained with antibodies against SYCP3 (red), Tel-FISH (green), and Lamin B (gray). An increased number of telomere (FISH) signals was observed at the inner site of the nucleus and did not co-localize with the NE (Lamin B) signals. White arrowheads indicate the inner telomeres. Zyg zygotene spermatocytes, Pac pachytene spermatocytes. b The number of internal telomeres in Stra8-Trf1−/− spermatocytes was increased compared with that of the control group. Zygotene to pachytene spermatocytes were counted for wild-type, whereas pachytene-like spermatocytes were counted for the Stra8-Trf1−/− spermatocytes. Trf1Flox/Flox, 1.17 ± 1.38; Stra8-Trf1−/−, 9.56 ± 1.62. The numbers of internal telomere (FISH) foci are presented as mean ± SEM. **P < 0.01. c An increased number of telomeres were detached from NE in the Trf1-deficient spermatocytes. Electron micrographs showing telomeres (red arrowheads) and inner NE (white arrows) in the Trf1Flox/Flox pachytene and Stra8-Trf1−/− pachytene-like nuclei; asterisks indicate membrane vesicles. Schematic illustrations represent the structures. AP attachment plate, CH chromatin, NE nuclear envelope, SC synaptonemal complex.