Abstract
Hydrocodone (HYD) is one of the most widely prescribed opioid analgesic drugs. Several neurotransmitters are involved in opioids relapse. Among these neurotransmitters, glutamate is suggested to be involved in opioid dependence and relapse. Glutamate is regulated by several glutamate transporters, including glutamate transporter 1 (GLT-1) and cystine/glutamate transporter (xCT). In this study, we investigated the effects of ceftriaxone (CEF) (200 mg/kg, i.p.), known to upregulate GLT-1 and xCT, on reinstatement to HYD (5 mg/kg, i.p.) using the conditioned place preference (CPP) paradigm in alcohol-preferring (P) rats. Animals were divided into three groups: 1) saline-saline group (SAL-SAL); 2) HYD-SAL group; and 3) HYD-CEF group. The CPP was conducted in four sessions: habituation phase, conditioning phase with HYD (i.p.) injections every other day for four sessions, extinction phase with CEF (i.p.) injections every other day for four sessions, and reinstatement phase with one priming dose of HYD. Time spent in the HYD-paired chamber after conditioning training was increased as compared to pre-conditioning. There was an increase in time spent in the HYD-paired chamber with one priming dose of HYD in the reinstatement test. HYD exposure downregulated xCT expression in the nucleus accumbens (NAc) and hippocampus (HIP), but no effects were observed in the dorsomedial prefrontal cortex (dmPFC) and amygdala (AMY). Importantly, CEF treatment attenuated the reinstatement effect of HYD and normalized xCT expression in the affected brain regions. These findings demonstrate that the attenuating effect of HYD reinstatement with CEF might be mediated through xCT.
Keywords: GLT-1, xCT, GLAST, Glutamate, Reinstatement, Hydrocodone, Opioids, CPP
Introduction
Opioids have long been used in pain management (1). However, their non-medical use has grown rapidly in the last few years. Hydrocodone (HYD) is one of the most widely used short-acting opioids; with over 136.7 million prescriptions in 2011 (2). HYD is a semi-synthetic opioid used for analgesic and antitussive purposes. However, data shows that the nonmedical use of HYD was one of the most common causes of emergency medical visits between 2004 and 2008 (3). HYD abuse liability and relative potency have been shown to be similar to those of oxycodone and hydromorphone (4). In addition, a study suggested that HYD has similar effects to those of oxycodone and morphine when administered intravenously (i.v) (5).
Relapse after a long period of abstinence is a major problem in the treatment of drug dependence (6). The high rate of relapse associated with opioids remains as one of the most challenging clinical problems in opioid dependence. It has been suggested that the glutamatergic system has regulatory effects on opioid dependence (7), withdrawal (8) and relapse in animals (9). Indeed, memantine (N-methyl-D-aspartate receptor blocker) attenuated reinstatement to morphine, while dopaminergic blockers failed (10). Studies have found an increase in extracellular glutamate concentration in the nucleus accumbens (NAc) with exposure to heroin (11), nicotine (12) and cocaine (13).
The extracellular glutamate is maintained via several glutamate transporters (also called the excitatory amino acid transporters, EAATs), including glutamate transporter 1 (GLT-1, EAAT2), glutamate/aspartate transporter (GLAST, EAAT1) and glutamate transporter (EAAT3). GLT-1 is a major glutamate transporter that regulates the uptake of the majority of glutamate (14, 15). It has been demonstrated that chronic exposure to morphine can lead to reduction of GLT-1 mRNA expression in the NAc, striatum, thalamus, and hippocampus (HIP) (7). Relapse to heroin was shown to be associated with increase in the extracellular glutamate concentration in the NAc (16). Thus, restoring the glutamate uptake may have beneficial therapeutic effect in attenuating opioid relapse. In regards to GLAST, this protein transports both glutamate and aspartate, and expressed mostly in the cerebellum and spinal cord (17). Several studies have suggested that the loss of morphine analgesic effect after repeated exposure to morphine might be due to reduction in GLAST expression as well as glutamate uptake in the spinal cord (18, 19). The EAAT3 is a neuronal glutamate transporter, expressed mainly in the HIP, basal ganglia and cerebellum (20). It has been shown that chronic exposure to morphine could downregulate the expression of EAAT3 in the HIP neuronal culture (21). In addition, recent report suggested that EAAT3 is important in morphine-induced conditioned place preference (CPP), but not in reinstatement (22).
Furthermore, cystine/glutamate transporter (xCT) is another transporter that regulates extracellular glutamate through the exchange of cystine with glutamate (14, 23, 24). Although there is less known about the role of xCT in opioid relapse, one study has demonstrated that restoring xCT function with N-acetylcysteine can attenuate heroin relapse in animals (25). N-acetylcysteine is known to improve the function of xCT, which might attenuate heroin relapse by increasing the glutamatergic tone on the pre-synaptic metabotropic glutamate receptor (mGluR2/3) (26). Thus, xCT might be a target candidate for the treatment of opioids dependence. Therefore, in this study, we investigated the effect of HYD reinstatement on the glial glutamate transporters such as GLT-1, xCT and GLAST.
In this study, we investigated the effects of HYD in alcohol-preferring (P) rats using the CPP paradigm. We used P rats due to the fact that they have higher density of mu opioid receptors than non-preferring (NP) rats (27). Similarly, others have found that alcohol-preferring Alko Alcohol (AA) rats express higher amount of opioid peptides and receptors as compared to alcohol-avoiding Alko Non-Alcohol (ANA) rats in several brain regions, including the NAc and ventral tegmental area (VTA) (28, 29). Also, AA rats have shown to be more susceptible to drug-induced behavioral sensitization in response to morphine than ANA rats (30). Therefore, high ethanol drinking rats may have a potential benefit over low ethanol drinking rats in testing opioids dependence and relapse. In this study, we tested a lower dose of HYD (5 mg/kg, i.p.) in P rats as sensitive bred to induce the conditioning and the reinstatement effects of HYD.
The β-lactam compounds, including ceftriaxone (CEF), have shown to attenuate drug-seeking in several drugs of abuse including methamphetamine (31), cocaine (32, 33), nicotine (34) and morphine (35). Moreover, in our laboratory, we have shown that CEF can reduce chronic alcohol drinking via upregulating both the expressions of GLT-1 and xCT in P rats (36, 37). Therefore, we hypothesized here that the administration of CEF during the extinction phase would attenuate HYD reinstatement by modulating these transporters. Thus, several important brain rewards regions involved in the glutamatergic transmission were investigated such as the dorsomedial prefrontal cortex (dmPFC), NAc, HIP and amygdala (AMY).
Materials and methods
Drugs
HYD (+)-bitartrate salt was purchased from Sigma-Aldrich (St. Louis, MO). CEF (Sandoz Inc., Princeton, NJ) was purchased from the pharmacy at the University of Toledo Medical Center. Saline (SAL) solution (0.9% NaCl) was used as vehicle to dissolve both drugs.
Animals and drug dosing
Male P rats were used to investigate the effects of HYD reinstatement on glutamate transporters: GLT-1, xCT and GLAST using the CPP paradigm. P rats were obtained from Indiana University, School of Medicine, Indianapolis, IN. Rats were housed in single plastic cages with free access to food and water. Housing room temperature was maintained at 21°C and humidity at 50%. Rats were on a 12:12-hour light-dark cycle throughout the whole study. All the proposed experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at The University of Toledo and adhered to the guidelines of the Institutional Animal Care and Use Committee of the National Institutes of Health and the Guide for the Care and Use of Laboratory Animals. The HYD dose was selected based previous study investigated HYD’s reward using CPP in rats (38) and the similarity between HYD and morphine rewarding effects (39). The use of CEF was based on previous studies that demonstrated that this drug attenuated morphine tolerance after its prolonged use (40). The dosing schedule of CEF was chosen based on recent study from our laboratory, which found that β-lactam compounds were able to attenuate cocaine reinstatement when given every other day during the extinction phase in P rats (33).
Apparatus
The apparatus consists of two chambers (40 cm × 40 cm × 40 cm) separated by smaller middle chamber (30 cm × 40 cm × 40 cm). The first chamber was distinguished with black and white horizontal stripes and textured floor. The second chamber was distinguished with black and white vertical stripes and smooth floor. The middle chamber was neutral. Time spent in either of the chambers was calculated by an observer who was blind to the experimental designs.
Experimental procedure
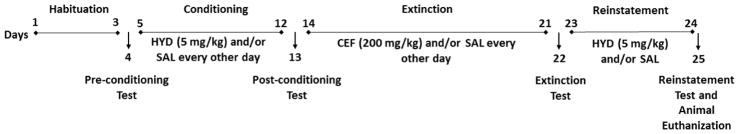
The CPP paradigm in this study was performed as described in a recent study from our laboratory (33). For acclimating purposes, animals were handled three days prior to starting the experiments. Rats were divided into three groups: 1) SAL-SAL group; 2) HYD-SAL group; and 3) HYD-CEF group in an unbiased manner as shown in (Table 1). The CPP was conducted in four phases: habituation, conditioning, extinction and reinstatement, as shown in (Figure 1). In the habituation phase, animals were allowed to explore the apparatus freely for 20 minutes a day, for three days to minimize stress and initial bias. On Day 4, animals were tested for initial preference (pre-conditioning test); animals were placed in the middle chamber with doors locked for three minutes. Then, both doors were opened for the animals to explore the apparatus for 20 minutes. The initial preference was calculated based on the time spent in the first and second chamber. Animals that showed more than 67% preference to one of the chambers were excluded from the study (41). In the conditioning phase, SAL (i.p) was administered in the SAL-SAL group and animals were placed in the assigned chamber alternatively for 30 minutes for a total of four sessions. Animals in the HYD-SAL group were given either HYD (5 mg/kg) or SAL (i.p.), alternatively, in the assigned chamber for 30 minutes for a total of four sessions. On Day 13, animals were tested for preference (post-conditioning test). In the extinction phase, SAL (i.p) was administered to the SAL-SAL group in the assigned chamber for 30 minutes for a total of four sessions. In the HYD-SAL group, animals were given SAL alternatively in the assigned chamber for 30 minutes for a total of four sessions. However, in the HYD-CEF group, animals were given CEF (200 mg/kg) or SAL (i.p.), alternatively, in the assigned chamber for 30 minutes for a total of four sessions. On Day 22, animals were tested for preference (extinction test). A 25% reduction in time spent in the HYD-paired chamber was set as a criteria for extinction, and any animal that did not meet that criteria was excluded, as was performed in previous studies from ours and others (31, 33). In the reinstatement phase, on Day 23, animals were challenged with one single dose of SAL (i.p.) in the SAL-SAL group and HYD (5 mg/kg, i.p.) in both groups (HYD-SAL and HYD-CEF) and placed in the assigned chamber for 30 minutes. Then, on Day 24, animals from all groups were given one dose of SAL (i.p.) and placed in the assigned chamber for 30 min. On Day 25, animals were tested for preference (reinstatement test) and euthanized on the same day.
Table 1.
Animal groups and treatment during the conditioning, extinction and reinstatement phases.
| Groups CPP phase |
SAL-SAL group | HYD-SAL group | HYD-CEF group |
|---|---|---|---|
| Conditioning | SAL | HYD/SAL | HYD/SAL |
| Extinction | SAL | SAL | CEF/SAL |
| Reinstatement | SAL | HYD/SAL | HYD/SAL |
Figure 1.
Timeline of the experimental procedure during the conditioning, extinction and reinstatement phases.
Brain tissue extraction
Animals were euthanized, and the brain samples were dissected after the reinstatement test on Day 25. The NAc (core and shell), dmPFC (cingulate cortex and prelimbic cortex), HIP (cornu ammonis, CA, subfield: CA1, CA2 and CA3) and AMY (central amygdala, basomedial amygdala and basolateral amygdala) were extracted using a cryostat machine (Leica CM1950). All brain regions were removed using the Brain Rat Atlas (42). All the samples were stored at −80°C for subsequent immunoblotting.
Immunoblots procedure
Samples were lysed using lysis buffer (50 mM Tris–HCl, 150 mM NaCl, 1 mM EDTA, 0.5% NP-40, 1% Triton, 0.1% SDS) with phosphatase and protease inhibitors. Glutamate transporters expression in this study represents the whole cell lysate of both membrane-bound and cytoplasmic protein fractions. Several studies have tested the effect of β-lactam compounds, using the whole cell lysate, on glutamate transporters in several drugs of abuse from ours and others (33, 43–48). Protein quantification was performed to measure the amount of protein in the lysed samples. Samples were loaded onto 10–15% Tris-glycine gel to separate the proteins via electrophoresis. Then, proteins were transferred to PVDF membranes. The membranes were then blocked with 3% fat-free milk in 10% Tris-buffered with Tween 20 (TBST) for 30–60 minutes. Membranes were incubated overnight at 4°C with the primary antibodies: anti Guinea pig GLT-1 (Millipore Sigma; 1:5000 dilution), anti-Rabbit xCT (Abcam; 1:1000 dilution), anti-Rabbit GLAST (Abcam; 1:5000 dilution) and anti-mouse β-tubulin (Covance;1:5000 dilution) as a loading control. Membranes were blocked with 3% fat-free milk in TBST for 30 minutes and incubated with the secondary antibodies for 90 minutes. Then, membranes were incubated with a Chemiluminescent kit (SuperSignal West Pico) and developed using X-Ray film processor (Konica SRX101A – Tabletop). Immunoblots were quantified using MCID Digital Imaging Software. The data were presented as a percentage of the ratio of the targeted proteins (GLT-1, xCT or GLAST)/β-tubulin. The control group was reported as 100% to measure the changes in the expression of GLT-1, xCT and GLAST after HYD and CEF treatment as performed in previous studies from ours and others (43, 49–52).
Statistical analyses
Time spent in the conditioning chambers after each phase in CPP were analyzed using two-way repeated measures ANOVA (Time × Chamber). Tukey’s post hoc test was used whenever a significant effect was found. The immunoblot data of GLT-1, xCT and GLAST were analyzed using one-way ANOVA followed by Newman-Keuls multiple comparison tests. All statistical analyses in this study were performed using GraphPad Prism with p < 0.05 as a level of significance.
Results
Effect of SAL administration on animal preference using CPP
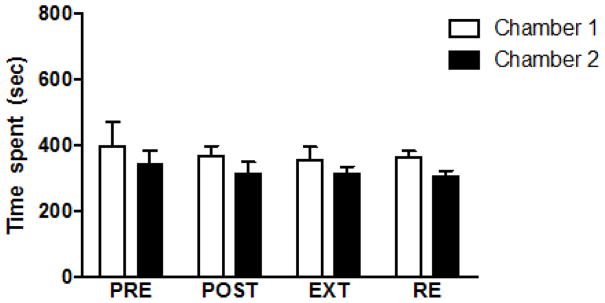
The effect of SAL administered alone was tested in CPP. Animals were habituated for three days to explore the CPP apparatus. Then, the initial preference was measured and considered as baseline. Animals were given SAL (i.p) during the conditioning, extinction and reinstatement phases every other day in chamber one and chamber two, alternately. Two-way repeated measures ANOVA showed no significant difference in time spent when SAL (i.p) was administered during conditioning, extinction and reinstatement (Figure 2). There were no significant effects of time [F (3, 18) = 1.289″, p = 0.3085], chamber effect [F (1, 6) = 1.194, p = 0.3165], nor time × chamber effect [F (3, 18) = 0.01702, p = 0.9969].
Figure 2.
Effect of SAL (i.p.) administration alone on CPP. No significant difference was found in time spent in preference test after the conditioning, extinction and reinstatement phases. Values are shown as means ± S.E.M (*p < 0.05) (n = 7). PRE = pre-conditioning, POST = postconditioning, EXT = extinction and RE = reinstatement.
Effect of CEF on HYD-induced reinstatement using CPP
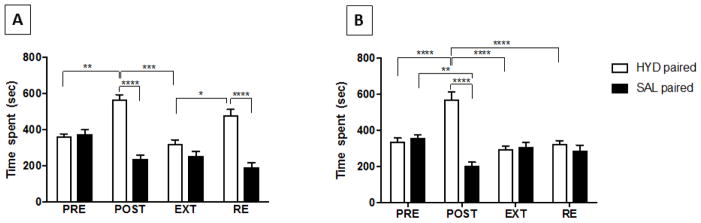
Time spent in the HYD-SAL group was analyzed using two-way repeated measures ANOVA (Figure 3A) with main significant effect of time [F (3, 18) = 18.49, p < 0.0001], significant effect of chamber [F (1, 6) = 399.7, p < 0.0001] and significant interaction between time × chamber [F (3, 18) = 15.87, p < 0.0001]. Tukey’s post hoc test showed significant increase in time spent in the HYD-paired chamber following conditioning training with HYD (5 mg/kg, i.p.) (p < 0.05, Figure 3A). This difference was eliminated in extinction. Time spent in the HYD-paired chamber was increased significantly after the reinstating the animals with one priming dose of HYD (5 mg/kg, i.p.) in the reinstatement phase (p < 0.05, Figure 3A).
Figure 3.
Time spent in the conditioning chamber during pre-conditioning, and in the postconditioning, extinction and reinstatement tests. Statistical analysis showed an increase in time spent in the HYD-paired chamber following conditioning compared to pre-conditioning in the HYD-SAL (A) and HYD-CEF (B) groups. Time spent in the HYD-paired chamber decreased in extinction in comparison with post-conditioning in the HYD-SAL and HYD-CEF groups. Time spent in the HYD-paired chamber increased in the reinstatement test in the HYD-SAL group, but not in the HYD-CEF group, in comparison to the extinction test. Values are shown as means ± S.E.M. *p < 0.05, **p < 0.01, and ****p < 0.0001. (n = 7–9 for each group). PRE = preconditioning, POST = post-conditioning, EXT = extinction and RE = reinstatement.
Two-way repeated measures ANOVA analysis in the HYD-CEF group (Figure 3B) showed a significant main effect of time [F (3, 24) = 13.73, p < 0.0001], a significant effect of chamber [F (1, 9) = 20.5, p = 0.0019] and a significant interaction between time × chamber [F (3, 24) = 30.73, p < 0.0001]. Tukey’s post hoc test showed significant increase in time spent in the HYD-paired chamber after conditioning training with HYD (5 mg/kg, i.p.) (p < 0.05, Figure 3B). This effect was again eliminated after extinction. However, no significant effect on time spent in the HYD-paired chamber was observed after reinstating the animals with one priming dose of HYD (5 mg/kg, i.p.) in the reinstatement phase (p > 0.05, Figure 3B).
Effect of CEF on the expression of GLT-1, xCT and GLAST in the NAc and dmPFC in HYD-induced reinstatement
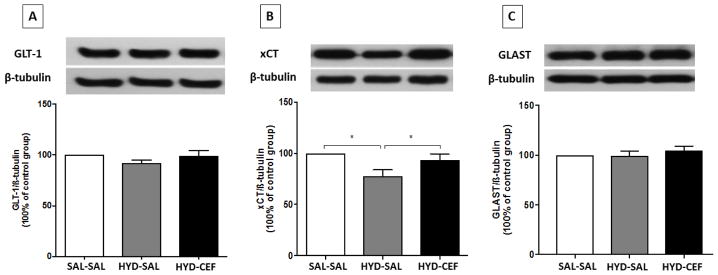
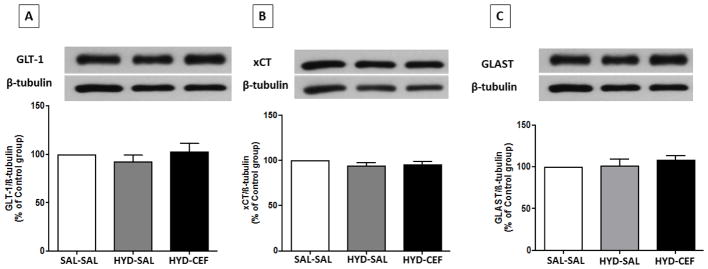
We investigated the effects of CEF on the expression of GLT-1, xCT and GLAST in the NAc and dmPFC in HYD reinstatement in P rats. One-way ANOVA showed no significant main effect on GLT-1 expression among the SAL-SAL, HYD-SAL and HYD-CEF groups in the NAc [F (2, 18) = 1.342, p = 0.2863, Figure 4A] or in the dmPFC [F (2, 18) = 0.7658, p = 0.4795, Figure 5A]. However, one-way ANOVA showed a significant main effect on xCT expression in the SAL-SAL, HYD-SAL and HYD-CEF groups in the NAc [F (2, 18) = 5.007, p = 0.0187, Figure 4B], but no effect in the dmPFC [F (2, 18) = 1.18, p = 0.3299, Figure 5B]. Further analysis with Newman-Keuls multiple comparison tests showed a significant downregulation in xCT expression in the HYD-SAL group compared to the SAL-SAL group in the NAc (p < 0.05, Figure 4B). However, statistical analysis showed a significant upregulation in xCT expression in the HYD-CEF group compared to the HYD-SAL group in the NAc (p < 0.05, Figure 4B). One way ANOVA showed no significant main effect on GLAST expression among the SAL-SAL, HYD-SAL and HYD-CEF groups in the NAc [F (2, 15) = 0.713, p = 0.5061, Figure 4C] and in the dmPFC [F (2, 15) = 0.8653, p = 0.4409, Figure 5C].
Figure 4.
Effects of HYD (5 mg/kg, i.p.) reinstatement and CEF (200 mg/kg, i.p.) on the expression of GLT-1, xCT and GLAST in the NAc. (A) Upper panel: immunoblots representing GLT-1 expression and β-tubulin. Lower panel: statistical analysis showed no significant difference among all groups. (B) Upper panel: immunoblots representing xCT expression and β-tubulin. Lower panel: statistical analysis showed significant downregulation in xCT expression in the HYD-SAL compared to the SAL-SAL group. However, statistical analysis showed upregulation of the HYD-CEF compared to the HYD-SAL. No statistical difference was found between the SAL-SAL and HYD-CEF groups. (C) Upper panel: immunoblots representing GLAST expression and β-tubulin. Lower panel: statistical analysis showed no significant difference among all groups. Values are shown as means± SEM (*p< 0.05) (n = 6–7 for each group).
Figure 5.
Effects of HYD (5 mg/kg, i.p.) reinstatement and CEF (200 mg/kg, i.p.) on the expression of GLT-1, xCT and GLAST in the dmPFC. (A) Upper panel: immunoblots representing GLT-1 expression and β-tubulin. Lower panel: statistical analysis showed no significant difference among all groups. (B) Upper panel: immunoblots representing xCT expression and β-tubulin. Lower panel: statistical analysis showed no significant difference between all groups. (C) Upper panel: immunoblots representing GLAST expression and β-tubulin. Lower panel: statistical analysis showed no significant difference among all groups. Values are shown as means± SEM (*p <0.05) (n = 6–7 for each group).
Effect of CEF on the expression of GLT-1, xCT and GLAST in the HIP and AMY in HYD-induced reinstatement
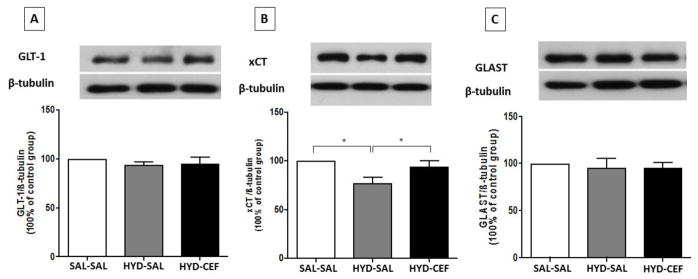
We investigated the effects of CEF on the expression of GLT-1, xCT and GLAST in the HIP and AMY in HYD reinstatement in P rats. One way ANOVA revealed no significant main effect on GLT-1 expression among the SAL-SAL, HYD-SAL and HYD-CEF groups in the HIP [F (2, 18) = 0.6305, p = 0.5437, Figure 6A] or the AMY [F (2, 18) = 0.6376, p = 0.5401, Figure 7A]. However, one-way ANOVA showed a significant main effect on xCT expression among the SAL-SAL, HYD-SAL and HYD-CEF groups in the HIP [F (2, 18) = 5.837, p = 0.0111, Figure 6B], but no effect in the AMY [F (2, 18) = 0.03411, p = 0.9665, Figure 7B]. In addition, Newman-Keuls multiple comparison tests revealed a significant downregulation in xCT expression in the HYD-SAL group compared to the SAL-SAL group in the HIP (p < 0.05, Figure 6B). However, statistical analysis showed a significant upregulation in xCT expression in the HYD-CEF group compared to the HYD-SAL group in the HIP (p < 0.05, Figure 6B). One way ANOVA showed no significant main effect on GLAST expression among the SAL-SAL, HYD-SAL and HYD-CEF groups in the HIP [F (2, 15) = 0.171, p = 0.8445, Figure 6C] and the AMY [F (2, 18) = 1.261, p = 0.3072, Figure 7C].
Figure 6.
Effects of HYD (5 mg/kg, i.p.) reinstatement and CEF (200 mg/kg, i.p.) on the expression of GLT-1, xCT and GLAST in the HIP. (A) Upper panel: immunoblots representing GLT-1 expression and β-tubulin. Lower panel: statistical analysis showed no significant difference among all groups. (B) Upper panel: immunoblots representing xCT expression and β-tubulin. Lower panel: statistical analysis showed significant downregulation in xCT expression in the HYD-SAL compared to the SAL-SAL group. However, statistical analysis showed upregulation of the HYD-CEF group compared to the HYD-SAL group. No statistical difference was found between the SAL-SAL and HYD-CEF groups. (C) Upper panel: immunoblots representing GLAST expression and β-tubulin. Lower panel: statistical analysis showed no significant difference among all groups. Values are shown as means± SEM (*p< 0.05) (n = 6–7 for each group).
Figure 7.
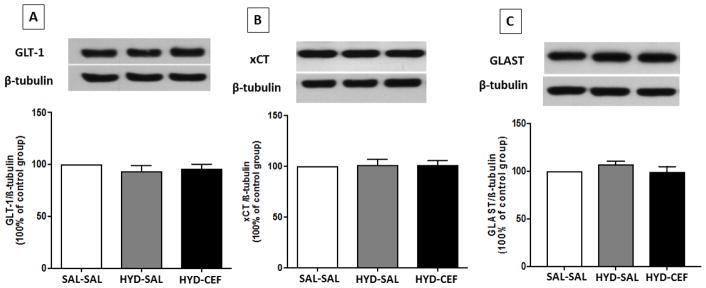
Effects of HYD (5 mg/kg, i.p.) reinstatement and CEF (200 mg/kg, i.p.) on the expression of GLT-1, xCT and GLAST in the AMY. (A) Upper panel: immunoblots representing GLT-1 expression and β-tubulin. Lower panel: statistical analysis showed no significant difference among all groups. (B) Upper panel: immunoblots representing xCT expression and β-tubulin. Lower panel: statistical analysis showed no significant difference among all groups. (C) Upper panel: immunoblots representing GLAST expression and β-tubulin. Lower panel: statistical analysis showed no significant difference among all groups. Values are shown as means ± SEM (*p < 0.05) (n = 6–7 for each group).
Discussion
The CPP paradigm has been used to measure opioid rewards, including heroin (53, 54), morphine (55, 56), HYD (38) and other drugs of abuse. In this study, the CPP paradigm was adopted from previous work in our laboratory on cocaine reinstatement (33). Several studies have focused on the association between the glutamatergic system and the reinstatement of morphine and heroin (10, 11). However, to the best of our knowledge, the association between HYD reinstatement and the glutamatergic system has not been thoroughly investigated, especially in P rats. In this study, we used HYD (5 mg/kg, i.p.) to produce the preference and reinstatement in P rats. Importantly, we found that HYD reinstatement was associated with downregulation of xCT in the NAc and HIP and these effects were attenuated with CEF treatment.
This study investigated the NAc (both core and shell), since this brain region is involved in opioid rewards (57), withdrawal (58) and tolerance (59). It has been found that blocking the mu-opioid receptor in the NAc diminished heroin rewards in rats (57). Moreover, high extracellular concentration of glutamate in the NAc core has been linked to heroin-seeking behavior and relapse (11). Also, it has been shown that injecting mGluR2/3 agonist (LY379268) into the NAc shell, but not NAc core, attenuated heroin-seeking behavior (60). Both NAc core and shell receive projections from the dmPFC [for review, see (61)]. It is important to note that glutamatergic projections from the PFC to NAc are suggested to be involved in cocaine- and heroin-seeking behavior (11, 62).
In this study, we also focused on the dmPFC, which included the cingulate cortex and prelimbic cortex. The activation of the cortex area in drug addiction was shown in neuroimaging studies during intoxication and craving, but not in withdrawal [for review see ref. (63)]. The mPFC appears to be involved in the acquisition of morphine, not the reinstatement (64). It has been suggested that there is a link between the PFC and cue-induced heroin-seeking (65). The cingulate cortex was reported to be activated following exposure to psychostimulant drugs such as cocaine (66, 67). In addition, it was reported that the cingulate cortex neural activity was altered in heroin users using the functional magnetic resonance imaging (68). The inactivation of the prelimbic cortex was shown to block the reinstatement of heroin (11, 69) and methamphetamine (70). Moreover, others have shown that activating the prelimbic cortex could facilitate heroin reinstatement (71). Moreover, the projections from the VTA to the NAc were suggested to be more important in opioid reinstatement as compared to the PFC (72), which might explain why this latter brain region was not affected in HYD reinstatement.
The HIP was investigated which included (CA1, CA2 and CA3). This brain region is involved in learning and memory function (73). Studies have reported that HIP is implicated in the association between the environmental context and unconditioned stimuli (foot-shock) (74, 75). A number of studies have demonstrated that the HIP is crucial for drug-seeking behavior (76–78). This brain region is believed to be associated with negative contextual experience associated with withdrawal from several drugs of abuse [for review, see ref (79). It has also been found that heroin can increase the amount of polysialic acid-neural cell adhesion molecule expression in glial cells, which could explain the damage found in the HIP area in postmortem heroin addicts (80). On the other hand, we investigated the AMY which included (central amygdala, basomedial amygdala and basolateral amygdala). It has been suggested that the AMY facilitates the drug reward and learning seeking behavior in rats (81). In addition, lesions in the AMY were shown to prevent cocaine reinstatement (82). Also, It has been found that inactivation of AMY using tetrodotoxin, potent neurotoxin, blocked the heroin-seeking behavior in rats (83).
Several studies have shown that regulation of glutamate homeostasis is critical in relapse to many drugs of abuse (84, 85). Glutamate is transported by GLT-1, which accounted for about 90% of glutamate clearance from the synaptic cleft (14, 15). CEF is known to produce its effects through GLT-1 upregulation. However, in this study, we found that CEF treatment was not associated with changes in GLT-1 expression in all tested brain areas. While no change in GLT-1 expression has been shown, there is a great possibility that CEF could improve the function of GLT-1 without changing its expression. Further studies are warranted to determine the activity of GLT-1 after CEF treatment in HYD-seeking behavior, for example, measuring extracellular glutamate concentrations using microdialysis technique. Another possibility is that the expression of GLT-1 might be different between sub-regions, where the effect in one area could be masked by the other. Future studies are still needed to determine subregion differential effects in GLT-1 expression. Studies have shown that chronic exposure to morphine reduced the expression of GLT-1 mRNA in the NAc, striatum, and thalamus (7). In addition, most of morphine studies, with regard to the glutamatergic system, were investigated in the cerebellum and spinal cord, which are more related to pain management, not relapse (86–88). Thus, more studies are required to investigate the effect of HYD with different doses and strains to examine the relationship between GLT-1 expression and other glutamate transporters in HYD exposure and reinstatement.
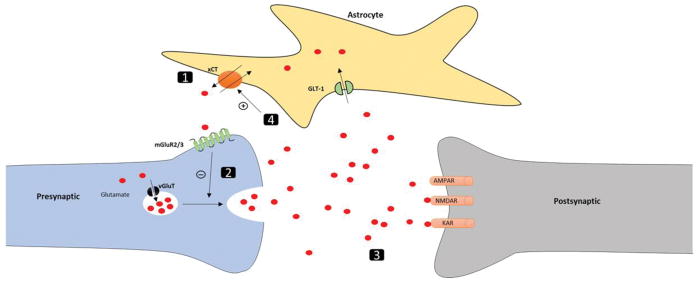
Although no changes were observed in GLT-1 expression with HYD reinstatement, we found that the xCT expression was downregulated in the NAc and HIP. Cellular mechanistic events involve xCT, GLT-1 and other glutamatergic receptors in several brain regions (e.g. NAc and HIP) to modulate HYD-seeking behavior are demonstrated in (Figure 8). To the best of our knowledge, little is known about the function of xCT in opioid dependence and relapse. However, it was reported that N-acetylcysteine can restore the function of xCT system and attenuate drug-seeking behavior in animals (89). Dysfunction of xCT can occur after cocaine self-administration, which was associated with a reduction in basal glutamate concentration in the NAc (90, 91). Similarly, repeated morphine exposure was associated with low basal glutamate concentration in the HIP in mice (92). It is important to note that restoring the xCT function can attenuate cocaine (90, 91) and heroin seeking behavior (25). Moreover, it has been shown that repeated administration of morphine can lead to behavioral sensitization in animals. Thus, morphine-sensitized rats have been shown to have high level of extracellular glutamate in the HIP, when they were challenged with morphine after prolonged abstinence (93). Therefore, restoring xCT function was assumed to improve the glutamatergic tone on mGluR2/3 and attenuate the seeking behavior as previously reported in cocaine (26). Here, we assumed that the downregulation of xCT expression in the NAc and HIP might be associated with reinstatement to HYD in P rats. Therefore, restoring xCT expression in these brain regions with CEF could, in part, attenuate HYD reinstatement (Figure 8).
Figure 8.
Proposed mechanistic events associated with changes in the xCT expression in the NAc and HIP for the attenuation of HYD-seeking behavior with CEF treatment. (1) HYD reinstatement was associated with reduction in the xCT expression in the NAc and HIP. This could be due to the effect of repeated exposure to HYD during the conditioning phase and HYD priming during the reinstatement phase. (2) The reduction in xCT expression may decrease the glutamatergic tone on mGluR2/3 and loss of the inhibitory mechanism on glutamate release. (3) High level of extracellular glutamate facilitates HYD reinstatement. (4) CEF treatment during the extinction phase increased xCT expression in the NAc and HIP, which could restore the glutamatergic tone on mGluR2/3. Restoring the inhibitory mechanism on glutamate release through mGluR2/3 could prevent high levels of extracellular glutamate and attenuate HYD reinstatement.
Furthermore, our study investigated another glial glutamate transporter called GLAST. GLAST expression was not changed in HYD reinstatement in all tested groups. This is in accordance with a previous study, which demonstrated that GLAST mRNA expression was not altered after morphine administration in the thalamus, hypothalamus, cerebral cortex, HIP, striatum, midbrain, cerebellum, and pons-medulla (7). Although other studies have shown that morphine administration is associated with reductions in GLAST and EAAT3 expression in the spinal cord (18, 19). In fact, GLAST is highly expressed in the cerebellum and spinal cord and less expressed in other brain regions (17) Together, these data suggest that GLAST is less involved. in HYD reinstatement.
The third-generation cephalosporin antibiotics can cross the blood brain barrier (BBB) (94). In addition, several studies have shown that CEF can also cross the BBB (95, 96) through a facilitated transport process (95). However, due to the fact that CEF has poor bioavailability when taken orally, CEF must be given through the parenteral route for maximum effects. CEF is highly bound to plasma proteins (95, 97), and most of CFE is eliminated in urine and biliary excretion [for review see Ref. (98)]. It is known that CEF can increase GLT-1 and xCT expression in the brain; therefore, different studies have investigated different possible mechanisms behind it. For instance, a study found that CEF treatment can facilitate the nuclear P65 translocation and activate the nuclear factor-κB (NF-κB) signaling pathway when tested in primary human fetal astrocytes (99). Moreover, it was reported that CEF increased the expression of NF-κB and the phosphorylation of Akt in the NAc and PFC in P rats (37). In addition, CEF was also found to activate the nuclear factor erythroid 2-related factor2 (Nrf2), which was suggested to increase the expression of xCT in the HIP cell line (100).
In this study, we used CEF (200 mg/kg, i.p.) to attenuate HYD reinstatement. Indeed, studies have found that CEF can attenuate reinstatement of morphine (35), heroin (16), methamphetamine (31), cocaine (32) and nicotine (34). In addition, several studies from our lab revealed that CEF (200 mg/kg, i.p.) attenuated chronic alcohol drinking in P rats (36, 37, 101, 102). Lower doses of CEF were not considered in this study due to the fact that it was administered every other day during the extinction phase. We have tested CEF at lower dose (50 mg/kg, i.p.) to attenuate cue-induced reinstatement to cocaine-seeking behavior (103). However, CEF at high dose (200 mg/kg, i.p.) attenuated cue-induced reinstatement to cocaine-seeking behavior and this effect was associated with upregulation of GLT-1 expression in the NAc and PFC. In fact, CEF at lower dose (50 mg/kg, i.p.) did not upregulate the expression of GLT-1, which suggests the non-attenuating effect of this dose in cocaine-seeking behavior. Furthermore, it has been suggested that CEF (200 mg/kg, i.p.) is equivalent to 13 g/day in clinical setting, where the normal dose of CEF is around 2 g/day (104). Also, it has been proposed that this dose could produce CNS concentration equivalent to the concentration needed to modulate GLT-1 expression (3.5 uM) in vitro (105). In general, high doses of CEF may cause unspecific adverse effects when used for a long period of time. However, several studies have used CEF to attenuate the reinstatement of other drugs of abuse for more than five consecutive days without reporting serious adverse effects (16, 31, 32, 34, 35).
In conclusion, this study showed for the first time that using HYD (5 mg/kg, i.p.) can produce conditioning and reinstatement effects in P rats using the CPP paradigm. Also, HYD reinstatement was associated with reduction in xCT expression in the NAc and HIP, but not in the dmPFC and AMY. Expressions of GLT-1 and GLAST were not affected in the HYD reinstatement. CEF (200 mg/kg, i.p.) prevented HYD reinstatement in P rats, in part, through modulating xCT expression in the NAc and HIP. Together, these data demonstrate that xCT has a vital role in HYD reinstatement in P rats.
Highlights.
Hydrocodone exposure downregulated xCT in nucleus accumbens and hippocampus.
Ceftriaxone attenuated the reinstatement effect of HYD.
Ceftriaxone attenuated hydrocodone-induced reduction in xCT.
Acknowledgments
This work was supported in part by Award Number R01AA019458 (Y.S.) from the National Institutes on Alcohol Abuse and Alcoholism and also by start-up funds from The University of Toledo. Fahad Alshehri was supported by a scholarship from Umm Al-Qura University, College of Pharmacy & Pharmaceutical Sciences, Makkah, Saudi Arabia. The authors would like to thank Mrs. Charisse Montgomery for editing this paper.
Footnotes
Author Contributions
FSA participated in study design and conceptualization, drafted and revised the manuscript, collected and analyzed the data. AYH participated in study design and conceptualization, collected the data and helped with the editing of the manuscript. YSA collected the data and helped with the editing of the manuscript. YS conceptualized and designed the study, critically revised the manuscript for intellectual content, and approved the final version of the manuscript.
Disclosure Statements
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ballantyne JC, LaForge SK. Opioid dependence and addiction during opioid treatment of chronic pain. Pain. 2007;129(3):235–55. doi: 10.1016/j.pain.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 2.Laxmaiah Manchikanti M, Standiford Helm I, MAJWJ Opioid epidemic in the United States. Pain physician. 2012;15:2150–1149. [PubMed] [Google Scholar]
- 3.Control CfD, Prevention U. Emergency department visits involving nonmedical use of selected prescription drugs - United States, 2004–2008. MMWR: Morbidity and mortality weekly report. 2010;59(23):705–9. [PubMed] [Google Scholar]
- 4.Walsh SL, Nuzzo PA, Lofwall MR, Holtman JR., Jr The relative abuse liability of oral oxycodone, hydrocodone and hydromorphone assessed in prescription opioid abusers. Drug Alcohol Depend. 2008;98(3):191–202. doi: 10.1016/j.drugalcdep.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoops WW, Hatton KW, Lofwall MR, Nuzzo PA, Walsh SL. Intravenous oxycodone, hydrocodone, and morphine in recreational opioid users: abuse potential and relative potencies. Psychopharmacology. 2010;212(2):193–203. doi: 10.1007/s00213-010-1942-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Brien CP. Drug addiction and drug abuse. The pharmacological basis of therapeutics. 1996;10:621–42. [Google Scholar]
- 7.Ozawa T, Nakagawa T, Shige K, Minami M, Satoh M. Changes in the expression of glial glutamate transporters in the rat brain accompanied with morphine dependence and naloxone-precipitated withdrawal. Brain research. 2001;905(1):254–8. doi: 10.1016/s0006-8993(01)02536-7. [DOI] [PubMed] [Google Scholar]
- 8.Tokuyama S, Wakabayashi H, Ho K. Direct evidence for a role of glutamate in the expression of the opioid withdrawal syndrome. European journal of pharmacology. 1996;295(2):123–9. doi: 10.1016/0014-2999(95)00645-1. [DOI] [PubMed] [Google Scholar]
- 9.Tahsili-Fahadan P, Carr GV, Harris GC, Aston-Jones G. Modafinil blocks reinstatement of extinguished opiate-seeking in rats: mediation by a glutamate mechanism. Neuropsychopharmacology. 2010;35(11):2203–10. doi: 10.1038/npp.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Do Couto BR, Aguilar M, Manzanedo C, Rodriguez-Arias M, Minarro J. NMDA glutamate but not dopamine antagonists blocks drug-induced reinstatement of morphine place preference. Brain research bulletin. 2005;64(6):493–503. doi: 10.1016/j.brainresbull.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 11.LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. Journal of Neuroscience. 2008;28(12):3170–7. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reid MS, Fox L, Ho LB, Berger SP. Nicotine stimulation of extracellular glutamate levels in the nucleus accumbens: neuropharmacological characterization. Synapse. 2000;35(2):129–36. doi: 10.1002/(SICI)1098-2396(200002)35:2<129::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 13.Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20(15):89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danbolt NC. Glutamate uptake. Progress in neurobiology. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276(5319):1699–702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 16.Shen H-w, Scofield MD, Boger H, Hensley M, Kalivas PW. Synaptic glutamate spillover due to impaired glutamate uptake mediates heroin relapse. The Journal of Neuroscience. 2014;34(16):5649–57. doi: 10.1523/JNEUROSCI.4564-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Storck T, Schulte S, Hofmann K, Stoffel W. Structure, expression, and functional analysis of a Na (+)-dependent glutamate/aspartate transporter from rat brain. Proceedings of the National Academy of Sciences. 1992;89(22):10955–9. doi: 10.1073/pnas.89.22.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao J, Sung B, Ji R-R, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. The Journal of neuroscience. 2002;22(18):8312–23. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tai Y-H, Wang Y-H, Wang J-J, Tao P-L, Tung C-S, Wong C-S. Amitriptyline suppresses neuroinflammation and up-regulates glutamate transporters in morphine-tolerant rats. Pain. 2006;124(1):77–86. doi: 10.1016/j.pain.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Maragakis NJ, Rothstein JD. Glutamate transporters in neurologic disease. Archives of neurology. 2001;58(3):365–70. doi: 10.1001/archneur.58.3.365. [DOI] [PubMed] [Google Scholar]
- 21.Guo M, Cao D, Zhu S, Fu G, Wu Q, Liang J, et al. Chronic exposure to morphine decreases the expression of EAAT3 via opioid receptors in hippocampal neurons. Brain Research. 2015;1628:40–9. doi: 10.1016/j.brainres.2015.03.037. [DOI] [PubMed] [Google Scholar]
- 22.Wan L, Bi J, Li J, Zuo Z. Glutamate transporter type 3 participates in maintaining morphine-induced conditioned place preference. Neuroscience. 2017;344:67–73. doi: 10.1016/j.neuroscience.2016.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bassi M, Gasol E, Manzoni M, Pineda M, Riboni M, Martín R, et al. Identification and characterisation of human xCT that co-expresses, with 4F2 heavy chain, the amino acid transport activity system x c. Pflügers Archiv European Journal of Physiology. 2001;442(2):286–96. doi: 10.1007/s004240100537. [DOI] [PubMed] [Google Scholar]
- 24.Bannai S, Sato H, Ishii T, Sugita Y. Induction of cystine transport activity in human fibroblasts by oxygen. Journal of Biological Chemistry. 1989;264(31):18480–4. [PubMed] [Google Scholar]
- 25.Zhou W, Kalivas PW. N-acetylcysteine reduces extinction responding and induces enduring reductions in cue-and heroin-induced drug-seeking. Biological psychiatry. 2008;63(3):338–40. doi: 10.1016/j.biopsych.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. Journal of Neuroscience. 2005;25(27):6389–93. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McBride WJ, Chernet E, McKinzie DL, Lumeng L, Li TK. Quantitative Autoradiography of Mu-Opioid Receptors in the CNS of Alcohol-Naive Alcohol-Preferring P and -Nonpreferring NP Rats. Alcohol. 1998;16(4):317–23. doi: 10.1016/s0741-8329(98)00021-4. [DOI] [PubMed] [Google Scholar]
- 28.de Waele JP, Kiianmaa K, Gianoulakis C. Distribution of the mu and delta opioid binding sites in the brain of the alcohol-preferring AA and alcohol-avoiding ANA lines of rats. Journal of Pharmacology and Experimental Therapeutics. 1995;275(1):518–27. [PubMed] [Google Scholar]
- 29.Marinelli PW, Kiianmaa K, Gianoulakis C. Opioid propeptide mRNA content and receptor density in the brains of AA and ANA rats. Life Sciences. 2000;66(20):1915–27. doi: 10.1016/s0024-3205(00)00517-8. [DOI] [PubMed] [Google Scholar]
- 30.Honkanen A, Mikkola J, Korpi ER, Hyytiä P, Seppälä T, Ahtee L. Enhanced morphine- and cocaine-induced behavioral sensitization in alcohol-preferring AA rats. Psychopharmacology. 1999;142(3):244–52. doi: 10.1007/s002130050886. [DOI] [PubMed] [Google Scholar]
- 31.Abulseoud OA, Miller JD, Wu J, Choi D-S, Holschneider DP. Ceftriaxone upregulates the glutamate transporter in medial prefrontal cortex and blocks reinstatement of methamphetamine seeking in a condition place preference paradigm. Brain research. 2012;1456:14–21. doi: 10.1016/j.brainres.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biological psychiatry. 2010;67(1):81–4. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammad AM, Alasmari F, Althobaiti YS, Sari Y. Modulatory effects of Ampicillin/Sulbactam on glial glutamate transporters and metabotropic glutamate receptor 1 as well as reinstatement to cocaine-seeking behavior. Behav Brain Res. 2017;332:288–98. doi: 10.1016/j.bbr.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alajaji M, Bowers M, Knackstedt L, Damaj M. Effects of the beta-lactam antibiotic ceftriaxone on nicotine withdrawal and nicotine-induced reinstatement of preference in mice. Psychopharmacology. 2013;228(3):419–26. doi: 10.1007/s00213-013-3047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan Y, Niu H, Rizak JD, Li L, Wang G, Xu L, et al. Combined action of MK-801 and ceftriaxone impairs the acquisition and reinstatement of morphine-induced conditioned place preference, and delays morphine extinction in rats. Neuroscience Bulletin. 2012;28(5):567–76. doi: 10.1007/s12264-012-1269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alhaddad H, Das SC, Sari Y. Effects of ceftriaxone on ethanol intake: a possible role for xCT and GLT-1 isoforms modulation of glutamate levels in P rats. Psychopharmacology. 2014;231(20):4049–57. doi: 10.1007/s00213-014-3545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao P, Sari Y. Effects of ceftriaxone on chronic ethanol consumption: a potential role for xCT and GLT1 modulation of glutamate levels in male P rats. Journal of Molecular Neuroscience. 2014;54(1):71–7. doi: 10.1007/s12031-014-0251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nazarian A, Are D, Tenayuca JM. Acetaminophen modulation of hydrocodone reward in rats. Pharmacology Biochemistry and Behavior. 2011;99(3):307–10. doi: 10.1016/j.pbb.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tenayuca JM, Nazarian A. Hydrocodone and morphine possess similar rewarding effects and reduce ERK and CREB phosphorylation in the nucleus accumbens. Synapse. 2012;66(10):918–22. doi: 10.1002/syn.21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rawls SM, Zielinski M, Patel H, Sacavage S, Baron DA, Patel D. Beta-lactam antibiotic reduces morphine analgesic tolerance in rats through GLT-1 transporter activation. Drug and alcohol dependence. 2010;107(2):261–3. doi: 10.1016/j.drugalcdep.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujio M, Nakagawa T, Sekiya Y, Ozawa T, Suzuki Y, Minami M, et al. Gene transfer of GLT-1, a glutamate transporter, into the nucleus accumbens shell attenuates methamphetamine-and morphine-induced conditioned place preference in rats. European Journal of Neuroscience. 2005;22(11):2744–54. doi: 10.1111/j.1460-9568.2005.04467.x. [DOI] [PubMed] [Google Scholar]
- 42.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Amsterdam; Boston: Academic Press/Elsevier; 2007. [Google Scholar]
- 43.Alshehri FS, Althobaiti YS, Sari Y. Effects of Administered Ethanol and Methamphetamine on Glial Glutamate Transporters in Rat Striatum and Hippocampus. Journal of Molecular Neuroscience. 2017;61(3):343–50. doi: 10.1007/s12031-016-0859-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alasmari F, Rao PSS, Sari Y. Effects of cefazolin and cefoperazone on glutamate transporter 1 isoforms and cystine/glutamate exchanger as well as alcohol drinking behavior in male alcohol-preferring rats. Brain Research. 2016;1634(Supplement C):150–7. doi: 10.1016/j.brainres.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Althobaiti YS, Alshehri FS, Almalki AH, Sari Y. Effects of ceftriaxone on glial glutamate transporters in Wistar rats administered sequential ethanol and methamphetamine. Frontiers in neuroscience. 2016:10. doi: 10.3389/fnins.2016.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hakami AY, Sari Y. β-Lactamase inhibitor, clavulanic acid, attenuates ethanol intake and increases glial glutamate transporters expression in alcohol preferring rats. Neuroscience Letters. 2017;657:140–5. doi: 10.1016/j.neulet.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J, John J, Langford D, Walker E, Ward S, Rawls SM. Clavulanic acid enhances glutamate transporter subtype I (GLT-1) expression and decreases reinforcing efficacy of cocaine in mice. Amino Acids. 2016;48(3):689–96. doi: 10.1007/s00726-015-2117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abulseoud OA, Camsari UM, Ruby CL, Kasasbeh A, Choi S, Choi D-S. Attenuation of ethanol withdrawal by ceftriaxone-induced upregulation of glutamate transporter EAAT2. Neuropsychopharmacology. 2014;39(7):1674. doi: 10.1038/npp.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Q, Tan Y. Nerve growth factor augments neuronal responsiveness to noradrenaline in cultured dorsal root ganglion neurons of rats. Neuroscience. 2011;193:72–9. doi: 10.1016/j.neuroscience.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 50.Wen Z-H, Wu G-J, Chang Y-C, Wang J-J, Wong C-S. Dexamethasone modulates the development of morphine tolerance and expression of glutamate transporters in rats. Neuroscience. 2005;133(3):807–17. doi: 10.1016/j.neuroscience.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 51.Simões AP, Duarte JA, Agasse F, Canas PM, Tomé AR, Agostinho P, et al. Blockade of adenosine A 2A receptors prevents interleukin-1β-induced exacerbation of neuronal toxicity through a p38 mitogen-activated protein kinase pathway. Journal of neuroinflammation. 2012;9(1):204. doi: 10.1186/1742-2094-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li J, Olinger A, Dassow M, Abel M. Up-regulation of GABAB receptor mRNA and protein in the hippocampus of cocaine-and lidocaine-kindled rats. Neuroscience. 2003;118(2):451–62. doi: 10.1016/s0306-4522(02)00995-8. [DOI] [PubMed] [Google Scholar]
- 53.Paul M, Dewey SL, Gardner EL, Brodie JD, Ashby CR. Gamma-vinyl GABA (GVG) blocks expression of the conditioned place preference response to heroin in rats. Synapse. 2001;41(3):219–20. doi: 10.1002/syn.1078. [DOI] [PubMed] [Google Scholar]
- 54.Ashby CR, Paul M, Gardner EL, Heidbreder CA, Hagan JJ. Acute administration of the selective D3 receptor antagonist SB-277011A blocks the acquisition and expression of the conditioned place preference response to heroin in male rats. Synapse. 2003;48(3):154–6. doi: 10.1002/syn.10188. [DOI] [PubMed] [Google Scholar]
- 55.Van Der Kooy D, Mucha RF, O’Shaughnessy M, Bucenieks P. Reinforcing effects of brain microinjections of morphine revealed by conditioned place preference. Brain research. 1982;243(1):107–17. doi: 10.1016/0006-8993(82)91124-6. [DOI] [PubMed] [Google Scholar]
- 56.Cavun S, Göktalay G, Millington WR. Glycyl-glutamine, an endogenous β-endorphin-derived peptide, inhibits morphine-induced conditioned place preference, tolerance, dependence, and withdrawal. Journal of Pharmacology and Experimental Therapeutics. 2005;315(2):949–58. doi: 10.1124/jpet.105.091553. [DOI] [PubMed] [Google Scholar]
- 57.Vaccarino FJ, Bloom FE, Koob GF. Blockade of nucleus accumbens opiate receptors attenuates intravenous heroin reward in the rat. Psychopharmacology. 1985;86(1):37–42. doi: 10.1007/BF00431681. [DOI] [PubMed] [Google Scholar]
- 58.Stinus L, Le Moal M, Koob GF. Nucleus accumbens and amygdala are possible substrates for the aversive stimulus effects of opiate withdrawal. Neuroscience. 1990;37(3):767–73. doi: 10.1016/0306-4522(90)90106-e. [DOI] [PubMed] [Google Scholar]
- 59.Schmidt BL, Tambeli CH, Barletta J, Luo L, Green P, Levine JD, et al. Altered nucleus accumbens circuitry mediates pain-induced antinociception in morphine-tolerant rats. Journal of Neuroscience. 2002;22(15):6773–80. doi: 10.1523/JNEUROSCI.22-15-06773.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006;31(10):2197. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moorman DE, James MH, McGlinchey EM, Aston-Jones G. Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain research. 2015;1628:130–46. doi: 10.1016/j.brainres.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. Journal of Neuroscience. 2001;21(21):8655–63. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159(10):1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hao Y, Yang J, Sun J, Qi J, Dong Y, Wu CF. Lesions of the medial prefrontal cortex prevent the acquisition but not reinstatement of morphine-induced conditioned place preference in mice. Neuroscience Letters. 2008;433(1):48–53. doi: 10.1016/j.neulet.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 65.Li Q, Wang Y, Zhang Y, Li W, Yang W, Zhu J, et al. Craving correlates with mesolimbic responses to heroin-related cues in short-term abstinence from heroin: an event-related fMRI study. Brain Res. 2012;1469:63–72. doi: 10.1016/j.brainres.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 66.Kilts CD, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. American Journal of Psychiatry. 2004;161(2):233–41. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- 67.Marhe R, Luijten M, van de Wetering BJM, Smits M, Franken IHA. Individual Differences in Anterior Cingulate Activation Associated with Attentional Bias Predict Cocaine Use After Treatment. Neuropsychopharmacology. 2013;38:1085. doi: 10.1038/npp.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y, Gong J, Xie C, Ye EM, Jin X, Song H, et al. Alterations in brain connectivity in three sub-regions of the anterior cingulate cortex in heroin-dependent individuals: Evidence from resting state fMRI. Neuroscience. 2015;284:998–1010. doi: 10.1016/j.neuroscience.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 69.Rogers J, Ghee S, See R. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151(2):579–88. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hiranita T, Nawata Y, Sakimura K, Anggadiredja K, Yamamoto T. Suppression of methamphetamine-seeking behavior by nicotinic agonists. Proceedings of the National Academy of Sciences. 2006;103(22):8523–7. doi: 10.1073/pnas.0600347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmidt ED, Voorn P, Binnekade R, Schoffelmeer AN, De Vries TJ. Differential involvement of the prelimbic cortex and striatum in conditioned heroin and sucrose seeking following long-term extinction. European Journal of Neuroscience. 2005;22(9):2347–56. doi: 10.1111/j.1460-9568.2005.04435.x. [DOI] [PubMed] [Google Scholar]
- 72.Self DW, Nestler EJ. Relapse to drug-seeking: neural and molecular mechanisms. Drug & Alcohol Dependence. 1998;51(1):49–60. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- 73.Postle BR. The hippocampus, memory, and consciousness. The neurology of consciousness: Cognitive neuroscience and neuropathology. 2009:326–38. [Google Scholar]
- 74.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256(5057):675. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 75.Phillips R, LeDoux J. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral neuroscience. 1992;106(2):274. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 76.Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to Cocaine-Seeking After Hippocampal Theta Burst Stimulation. Science. 2001;292(5519):1175–8. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- 77.Black YD, Green-Jordan K, Eichenbaum HB, Kantak KM. Hippocampal memory system function and the regulation of cocaine self-administration behavior in rats. Behavioural brain research. 2004;151(1–2):225–38. doi: 10.1016/j.bbr.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 78.Yang Y, Zheng X, Wang Y, Cao J, Dong Z, Cai J, et al. Stress enables synaptic depression in CA1 synapses by acute and chronic morphine: possible mechanisms for corticosterone on opiate addiction. Journal of Neuroscience. 2004;24(10):2412–20. doi: 10.1523/JNEUROSCI.5544-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weber M, Modemann S, Schipper P, Trauer H, Franke H, Illes P, et al. Increased polysialic acid neural cell adhesion molecule expression in human hippocampus of heroin addicts. Neuroscience. 2006;138(4):1215–23. doi: 10.1016/j.neuroscience.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 81.Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward the role of amygdala-ventral striatal subsystems. Annals of the New York Academy of Sciences. 1999;877(1):412–38. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- 82.Whitelaw RB, Markou A, Robbins TW, Everitt BJ. Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcement. Psychopharmacology. 1996;127(1–2):213–24. [PubMed] [Google Scholar]
- 83.Fuchs RA, See RE. Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology. 2002;160(4):425–33. doi: 10.1007/s00213-001-0997-7. [DOI] [PubMed] [Google Scholar]
- 84.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nature reviews Neuroscience. 2009;10(8):561. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 85.Knackstedt LA, Kalivas PW. Glutamate and reinstatement. Current opinion in pharmacology. 2009;9(1):59–64. doi: 10.1016/j.coph.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Niederberger E, Schmidtko A, Rothstein J, Geisslinger G, Tegeder I. Modulation of spinal nociceptive processing through the glutamate transporter GLT-1. Neuroscience. 2003;116(1):81–7. doi: 10.1016/s0306-4522(02)00547-x. [DOI] [PubMed] [Google Scholar]
- 87.Tai Y-H, Wang Y-H, Tsai R-Y, Wang J-J, Tao P-L, Liu T-M, et al. Amitriptyline preserves morphine’s antinociceptive effect by regulating the glutamate transporter GLAST and GLT-1 trafficking and excitatory amino acids concentration in morphine-tolerant rats. Pain. 2007;129(3):343–54. doi: 10.1016/j.pain.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 88.Gunduz O, Oltulu C, Buldum D, Guven R, Ulugol A. Anti-allodynic and anti-hyperalgesic effects of ceftriaxone in streptozocin-induced diabetic rats. Neuroscience letters. 2011;491(1):23–5. doi: 10.1016/j.neulet.2010.12.063. [DOI] [PubMed] [Google Scholar]
- 89.Baker DA, McFARLAND K, Lake RW, Shen H, Toda S, Kalivas PW. N-Acetyl Cysteine-Induced Blockade of Cocaine-Induced Reinstatement. Annals of the New York Academy of Sciences. 2003;1003(1):349–51. doi: 10.1196/annals.1300.023. [DOI] [PubMed] [Google Scholar]
- 90.Baker DA, McFarland K, Lake RW, Shen H, Xing-Chun T, Toda S, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nature neuroscience. 2003;6(7):743. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 91.Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, et al. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. Journal of Neuroscience. 2007;27(51):13968–76. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guo M, Xu N-J, Li Y-T, Yang J-Y, Wu C-F, Pei G. Morphine modulates glutamate release in the hippocampal CA1 area in mice. Neuroscience Letters. 2005;381(1):12–5. doi: 10.1016/j.neulet.2005.01.071. [DOI] [PubMed] [Google Scholar]
- 93.Farahmandfar M, Karimian SM, Zarrindast M-R, Kadivar M, Afrouzi H, Naghdi N. Morphine sensitization increases the extracellular level of glutamate in CA1 of rat hippocampus via μ-opioid receptor. Neuroscience letters. 2011;494(2):130–4. doi: 10.1016/j.neulet.2011.02.074. [DOI] [PubMed] [Google Scholar]
- 94.Fekety FR. Safety of parenteral third-generation cephalosporins. The American journal of medicine. 1990;88(4):S38–S44. doi: 10.1016/0002-9343(90)90326-9. [DOI] [PubMed] [Google Scholar]
- 95.Spector R. Ceftriaxone transport through the blood-brain barrier. The Journal of infectious diseases. 1987;156(1):209–11. doi: 10.1093/infdis/156.1.209. [DOI] [PubMed] [Google Scholar]
- 96.Spector R. Ceftriaxone pharmacokinetics in the central nervous system. Journal of Pharmacology and Experimental Therapeutics. 1986;236(2):380–3. [PubMed] [Google Scholar]
- 97.Steele RW, Eyre LB, Bradsher RW, Weinfeld RE, Patel IH, Spicehandler J. Pharmacokinetics of ceftriaxone in pediatric patients with meningitis. Antimicrobial agents and chemotherapy. 1983;23(2):191–4. doi: 10.1128/aac.23.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Richards D, Heel R, Brogden R, Speight T, Avery G. Ceftriaxone. Drugs. 1984;27(6):469–527. doi: 10.2165/00003495-198427060-00001. [DOI] [PubMed] [Google Scholar]
- 99.Lee S-G, Su Z-Z, Emdad L, Gupta P, Sarkar D, Borjabad A, et al. Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. Journal of Biological Chemistry. 2008;283(19):13116–23. doi: 10.1074/jbc.M707697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lewerenz J, Albrecht P, Tien MLT, Henke N, Karumbayaram S, Kornblum HI, et al. Induction of Nrf2 and xCT are involved in the action of the neuroprotective antibiotic ceftriaxone in vitro. Journal of neurochemistry. 2009;111(2):332–43. doi: 10.1111/j.1471-4159.2009.06347.x. [DOI] [PubMed] [Google Scholar]
- 101.Das SC, Yamamoto BK, Hristov AM, Sari Y. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology. 2015;97:67–74. doi: 10.1016/j.neuropharm.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL. Ceftriaxone, a Beta-Lactam Antibiotic, Reduces Ethanol Consumption in Alcohol-Preferring Rats. Alcohol and Alcoholism. 2011;46(3):239–46. doi: 10.1093/alcalc/agr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. The Journal of Neuroscience. 2009;29(29):9239–43. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rasmussen BA, Baron DA, Kim JK, Unterwald EM, Rawls SM. β-Lactam antibiotic produces a sustained reduction in extracellular glutamate in the nucleus accumbens of rats. Amino Acids. 2011;40(2):761–4. doi: 10.1007/s00726-010-0589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, et al. β-Lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433(7021):73–7. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]