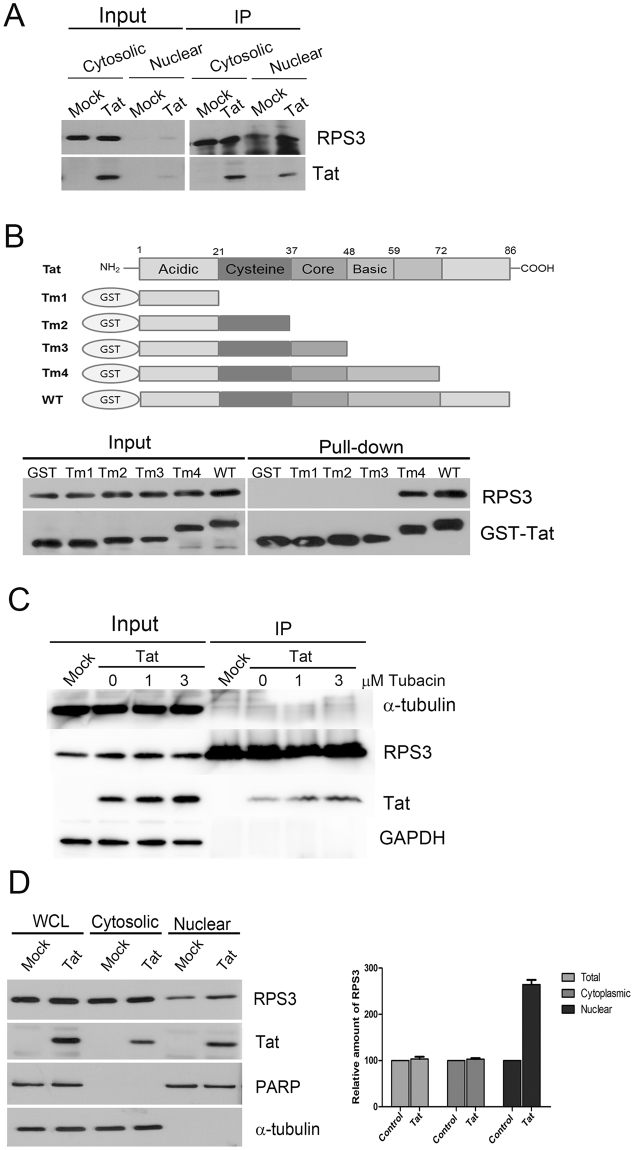
Figure 1.
HIV-1 Tat interacts with RPS3 and induces nuclear accumulation of RPS3. (A) Co-immunoprecipitation of Tat and RPS3 in HEK 293FT cells transfected with the control or Tat expression construct. Results are representative of three independent experiments. (B) Tat binds to RPS3 through its basic domain. Expression constructs encoding GST-Tat deletion mutants were transfected into 293FT cells, GST-Tat deletion mutants were pulled down with glutathione agarose beads, and co-precipitation of RPS3 was examined by immunoblotting. (C) Acetylation of Tat at K28 may increase its interaction with RPS3. 293FT cells were transfected with the Tat expression construct and incubated for 24 h in the presence of 0, 1, or 3 μM tubacin, an HDAC6 inhibitor. Thereafter, 500 μg of each cell extract was subjected to immunoprecipitation with an anti-RPS3 antibody. Bound proteins were analyzed by immunoblotting with anti-Tat, anti-α-tubulin, anti-GAPDH, and anti-RPS3 antibodies. (D) HIV-1 Tat induces nuclear accumulation of RPS3 in 293FT cells. 293FT cells were transfected with the Tat expression construct and incubated for 16 h. Nuclear and cytosolic fractions were separated and analyzed by western blotting with anti-α-tubulin, anti-PARP, anti-RPS3, and anti-Tat antibodies (left panel). WCL: whole cell lysate. Blots were scanned and the intensity of the RPS3 band was quantified by ImageJ software and normalized against that in the control sample. Data represent the mean ± SEM of four independent experiments (right panel).