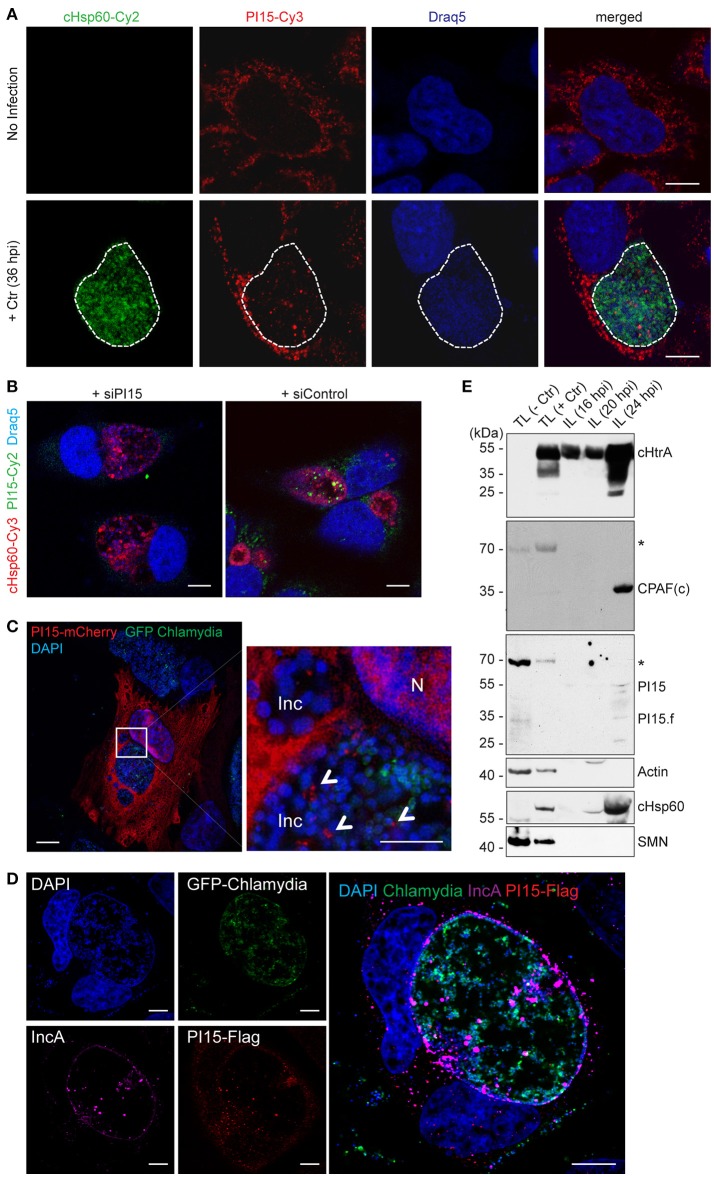
Figure 2.
PI15 is recruited into the chlamydial inclusion. (A) PI15 is localized within the chlamydial inclusion. HeLa cells were either left uninfected or infected with C. trachomatis (Ctr) for 24 h. Cells were immunostained using PI15 or Chlamydia-specific Hsp60 (cHsp60) antibodies. Draq5 was used to stain DNA. The chlamydial inclusion is marked with a white dotted line. (B) Validation of the PI15 antibody by RNAi. HeLa cells were either transfected with siRNAs against PI15 or control siRNAs. 72 h post transfection, cells were infected with C. trachomatis. Cells were immunostained as mentioned above. (C) PI15 localizes in the lumen of the chlamydial inclusion. HeLa cells were transiently transfected with a mCherry-tagged PI15 construct. 48 h after transfection, cells were infected with C. trachomatis expressing GFP. Cells were fixed 30 h post infection and stained with DAPI. SIM microscopy was used to study the localization of mCherry-PI15 within the chlamydial inclusion. Inc, inclusion; N, Nucleus. (D) Recruitment of Flag-tagged PI15 to the chlamydial inclusion. HeLa cells were transiently transfected with a Flag-tagged PI15 construct for 48 h and then infected with C. trachomatis expressing GFP. Cells were fixed and immunostained using antibodies against Flag and chlamydial IncA. (E) PI15 was detected within purified chlamydial inclusions. HeLa cells were infected with C. trachomatis for different time intervals. The chlamydial inclusion was purified from the cells and protein lysates (IL) were prepared from purified inclusions. Total cell lysates (TL) from uninfected and 24 h infected cells were used as control. Actin and survival motor neuron (SMN) were detected as markers for cytosol and nuclei to evaluate inclusion purity. hpi, hours post infection. *, non-specific band. Scale bars in all panels, 10 μM.