Table 3.
Disulfuration with carbon nucleophiles a,b
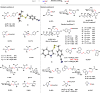
|
aStandard conditions A: NuH (0.22 mmol, 1.1 equiv), 2 (0.2 mmol, 1 equiv), B(C6F5)3 (0.01 mmol, 5 mol%) and 4-MeOPy (0.01 mmol, 5 mol%) were added to DCE (0.25 mL) at r.t. for 22 h. Standard conditions B: NuH (0.3 mmol, 1.5 equiv), 2 (0.2 mmol, 1 equiv) and B(C6F5)3 (0.01 mmol, 5 mol%) were added to PhMe (0.5 mL) at 0 °C for 24 h. Standard conditions C: NuH (0.3 mmol, 1.5 equiv), 2 (0.2 mmol, 1 equiv) and MeSO3H (0.02 mmol, 10 mol%) were added to tAmylOH (0.5 mL) at 0 °C for 5–24 h
bIsolated yields
cr.t. was instead of 0 °C
dB(C6F5)3 (0.002 mmol, 1 mol%) was used
eB(C6F5)3 (0.01 mmol, 0.2 mol%) was used
fB(C6F5)3 (0.004 mmol, 2 mol%) were added to PhMe (0.25 mL) at r.t. for 24 h
gNuH (0.22 mmol, 1.1 equiv), 2 (0.2 mmol, 1 equiv) and B(C6F5)3 (0.004 mmol, 2 mol%) were added to PhMe (0.25 mL) at 0 °C for 24 h. Ar = 4-CNC6H4
