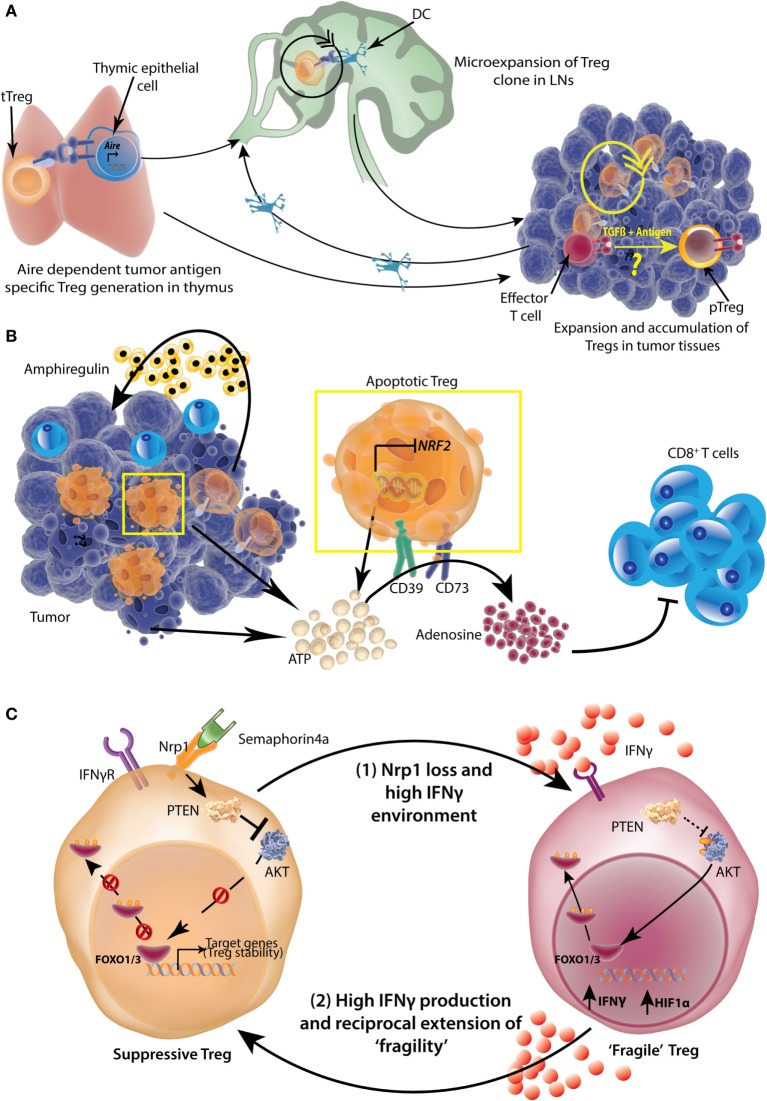
Figure 7.
Origin, accumulation, and functional potentiation of tumor-infiltrating regulatory T-cells (Tregs). (A) Tumor-specific antigens can be expressed by thymic epithelial cells in an Aire-dependent manner, which then select the tumor antigen-specific Tregs. These Tregs then expand in tumor draining lymph nodes (LNs) with the help of dendritic cells (DCs). Tregs can also be directly recruited to tumors and undergo expansion there. While intratumoral conversion of effector T-cells to pTregs is likely, the extent to which this occurs under physiological conditions is not completely understood. (B) A high number of TI-Tregs are apoptotic because of suppressed expression of Nrf2. These Tregs as well as dying tumor cells release copious amounts of ATP, which is converted to adenosine by Treg ectoenzymes CD39 and CD73 in a sequential manner. The resulting adenosine is highly suppressive to tumor-infiltrating CD8+ effector T-cells. Further, Tregs also produce Amphiregulin in certain tumor types, which help in tumor progression. (C) More than 50% of TI-Tregs express surface Neuropilin1 (Nrp1), which is a receptor for Semaphorin 4a. Upon ligation with Semaphorin4a, Nrp1 activates lipid phosphatase Phosphatase and tensin homolog (PTEN) which promptly dephosphorylates AKT rendering its sequestration in cytosol and nuclear retention of Foxo1/3 transcription factors which help in stabilization and survival of Tregs. A loss of Nrp1 renders Treg highly susceptible to IFNγ and such Tregs also produce high amount of IFNγ and HIF1α (“Fragile” Tregs). The resultant high IFNγ environment, reciprocally, can induce “fragility” in other Nrp1-sufficient Tregs as well, setting up a vicious cycle.