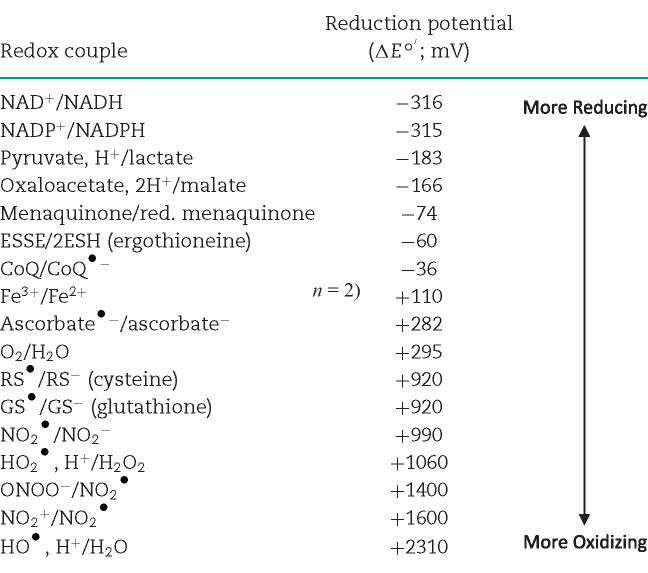
Table 1.
Redox potentials of biologically relevant redox couples.
|
Redox couples are ordered by increasing reduction potential (ΔEo΄). ΔEo΄ values were adjusted from the reduction potential under standard conditions to reflect the potential at a pH = 7 using the Nernst equation, as H+ is an important constituent of many of the couples in question. It is important to recognize that these values do not directly reflect ΔE values in vivo, as various conditions, including temperature and concentration of reactants can vary dramatically. ΔEo΄ values shown were originally reported by Schafer and Buettner (2001) and Halliwell and Gutteridge (2008).
