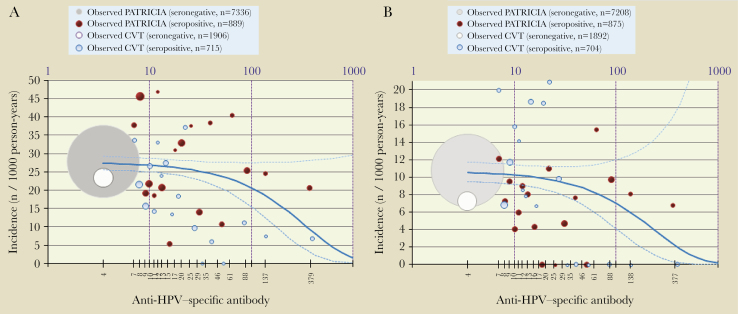
Figure 2.
Quantitative Poisson regression model showing relationship between initial HPV-18 antibody level and incident HPV-18 infection (A) and HPV-18–associated ASC-US+ (B). TVC-E control arm: 15–25 years old, who reported ever having had sexual intercourse. Infection: the adjusted model-predicted HPV-18 antibody titers associated with a 90% risk reduction of incident infection were 622 EU/mL (95% CI, 334–3838). ASU-CS+: the adjusted model-predicted HPV-18 antibody titers associated with a 90% risk reduction of ASC-US+ were 480 EU/mL (95% CI, 217–not determined). The dot size is proportional to the number of subjects; gray/white dot represents all seronegative subjects, and red/blue dots represent approximately 5-percentile classes of seropositive subjects. The solid blue line corresponds to the Poisson regression model (the dotted lines are 95% confidence limits). Abbreviations: ASC-US+, atypical squamous cells of undetermined significance or greater; CI, confidence interval; CVT, Costa Rica Vaccine Trial; HPV, human papillomavirus; n, number of subjects in a given category; PATRICIA, The PApilloma TRIal against Cancer In young Adults; TVC-E, total vaccinated cohort for efficacy.