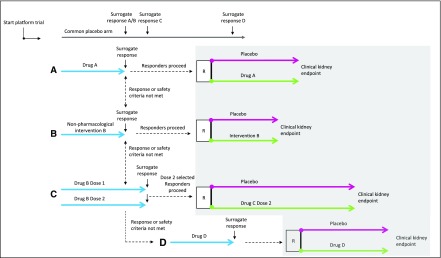
Figure 1.
Design of a hypothesized platform in kidney disease. After the platform is launched, patients are screened and randomly assigned to treatment with intervention (A–C) or placebo. The intervention can be a lifestyle intervention or pharmacologic intervention. A common placebo arm is used in this example, which can increase clinical trial efficiency, although challenges with respect to blinding and multiple end point assessments have to be appropriately addressed. During the first phase of the hypothesized platform, the effect of the intervention is tested on a surrogate outcome to select promising therapies and/or optimal doses for a confirmatory clinical end point trial. Individuals who respond to the intervention according to a predefined surrogate response threshold and who tolerate the intervention will proceed to the confirmatory trial (gray shaded area); they will be randomly assigned to placebo or active therapy and followed for the remainder of the trial until the prespecified number of clinical end points is accrued. Individuals who do not show a predefined surrogate response (nonresponders) or in whom tolerability or safety issues arise will not be randomized to their first assigned intervention; they will rotate and be exposed to therapy with other interventions available in the platform. New drugs can enter the platform at any time (intervention D in the hypothesized platform), whereas futile therapies or doses considered to be inefficacious can be dropped from the platform. R, randomization.